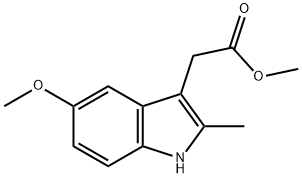
methyl 5-methoxy-2-methyl-1H-indole-3-acetate synthesis
- Product Name:methyl 5-methoxy-2-methyl-1H-indole-3-acetate
- CAS Number:7588-36-5
- Molecular formula:C13H15NO3
- Molecular Weight:233.26

67-56-1
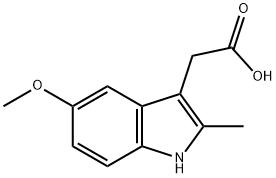
2882-15-7

7588-36-5
General method: 5-methoxy-2-methyl-3-indoleacetic acid (1 eq.) was dissolved with BOP-Cl (1 eq.) in anhydrous CH2Cl2 (3 mL/mmol), triethylamine (2 eq.) was added, and the reaction was carried out for 5 min at room temperature. Subsequently, anhydrous methanol (0.14 mL/mmol) was added and stirred overnight at room temperature. After completion of the reaction, the reaction mixture was diluted with dichloromethane (12 mL/mmol), washed with water (2 x 6 mL/mmol), dried over anhydrous Na2SO4 and filtered. The filtrate was concentrated under reduced pressure, and the resulting crude product was purified by rapid chromatography on silica gel (gradient elution with ethyl acetate/hexane) to afford methyl 5-methoxy-2-methyl-1H-indole-3-acetate, which was initially a yellow viscous oil, and crystallized and solidified after inoculation and refrigeration.

67-56-1
790 suppliers
$9.00/25ml

2882-15-7
161 suppliers
$12.00/100mg

7588-36-5
77 suppliers
$21.00/100mg
Yield:7588-36-5 100%
Reaction Conditions:
Stage #1: 5-Methoxy-2-methylindole-3-acetic acidwith bis-(2-oxo-3-oxazolidinyl)phosphoryl chloride;triethylamine in dichloromethane at 20; for 0.0833333 h;
Stage #2: methanol in dichloromethane at 20;
Steps:
General Procedure A_variant1:
General procedure: A reaction mixture containing the indoleacetic acid derivative (1 equivalent) and BOP-Cl (1 equivalent) in 3 mL/mmol of anhydrous CH2Cl2 is treated with triethylamine (2 equivalents) and aged at ambient temperature for 5 min. The mixture is combined with anhydrous methanol (0.14 mL/mmol) and then continuously stirred overnight at room temperature. Following dilution with dichloromethane (12 mL/mmol), the organic solution is washed with water (2x6 mL/mmol), dried over Na2SO4, and filtered. The organic filtrate is collected and concentrated in vacuo and the crude ester is purified by flash chromatography on silica gel (ethyl acetate/hexane gradient) to afford the title compound at first as viscous yellow oil, which stably crystallizes upon seeding and subsequent cold storage.
References:
Liedtke, Andy J.;Marnett, Lawrence J.;Kim, Kwangho;Stec, Donald F.;Sulikowski, Gary A. [Tetrahedron,2012,vol. 68,# 48,p. 10049 - 10058,10] Location in patent:supporting information Liedtke, Andy J.;Kim, Kwangho;Stec, Donald F.;Sulikowski, Gary A.;Marnett, Lawrence J. [Tetrahedron,2012,vol. 68,# 48,p. 10049 - 10058] Location in patent:supporting information

2882-15-7
161 suppliers
$12.00/100mg

7588-36-5
77 suppliers
$21.00/100mg
624-45-3
230 suppliers
$10.00/5g
19501-58-7
396 suppliers
$7.00/250mg

7588-36-5
77 suppliers
$21.00/100mg

2882-15-7
161 suppliers
$12.00/100mg
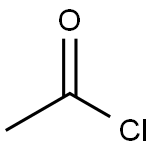
75-36-5
589 suppliers
$17.92/100G

7588-36-5
77 suppliers
$21.00/100mg
![Pentanoic acid, 4-[2-(4-methoxyphenyl)hydrazinylidene]-, methyl ester](/CAS/20210305/GIF/53258-38-1.gif)
53258-38-1
0 suppliers
inquiry

7588-36-5
77 suppliers
$21.00/100mg