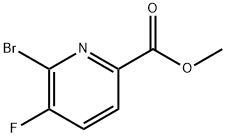
Methyl 6-bromo-5-fluoropyridine-2-carboxylate synthesis
- Product Name:Methyl 6-bromo-5-fluoropyridine-2-carboxylate
- CAS Number:1210419-26-3
- Molecular formula:C7H5BrFNO2
- Molecular Weight:234.02

67-56-1
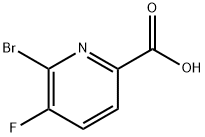
1052714-46-1

1210419-26-3
Synthesis of methyl 6-bromo-5-fluoro-pyridinecarboxylate: Concentrated sulfuric acid (4.2 eq.) was slowly added to a methanol (0.2 M) solution of 6-bromo-5-fluoro-2-pyridinecarboxylic acid (1.0 eq.) and the reaction mixture was stirred at room temperature. The progress of the reaction was monitored by LC/MS and the reaction was completed after about 2 hours. The reaction mixture was diluted with ethyl acetate and saturated aqueous sodium bicarbonate solution was added slowly to quench the reaction. The mixture was transferred to a partition funnel and extracted with ethyl acetate. The organic phases were combined, dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to afford methyl 6-bromo-5-fluoropyridinecarboxylate as a white solid (yield >99%). The product was characterized by LC/MS: m/z = 233.9/235.9 ([M+H]+), retention time Rt = 0.69 min.

67-56-1
790 suppliers
$7.29/5ml-f

1052714-46-1
125 suppliers
$10.00/100mg

1210419-26-3
72 suppliers
$65.00/50mg
Yield: 99%
Reaction Conditions:
with sulfuric acid at 20; for 2 h;
Steps:
Synthesis of methyl 6-bromo-5-fluoropicolinate
Synthesis of methyl 6-bromo-5-fluoropicolinate To a solution of 6-bromo-5-fluoropicolinic acid (1.0 equiv.) in methanol (0.2 M) was added H2SO4 (4.2 equiv.) and the reaction was stirred at room temperature for two hours. Upon completion of the reaction as monitored by LC/MS, the reaction was diluted with ethyl acetate and quenched slowly with saturated aqueous NaHCO3. The reaction was poured into a separatory funnel and extracted with ethyl acetate. The organic phase was dried with magnesium sulfate, filtered, and concentrated in vacuo to provide methyl 6-bromo-5-fluoropicolinate as a white solid (>99%). LC/MS=233.9/235.9 (M+H), Rt=0.69 min.
References:
BURGER, Matthew T.;HAN, Wooseok;LAN, Jiong;NISHIGUCHI, Gisele US2010/56576, 2010, A1 Location in patent:Page/Page column 48

1052714-46-1
125 suppliers
$10.00/100mg

1210419-26-3
72 suppliers
$65.00/50mg
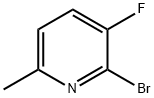
374633-36-0
198 suppliers
$9.00/250mg

1210419-26-3
72 suppliers
$65.00/50mg