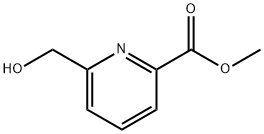
METHYL-6-HYDROXYMETHYL-2-CARBOXYLATE PYRIDINE synthesis
- Product Name:METHYL-6-HYDROXYMETHYL-2-CARBOXYLATE PYRIDINE
- CAS Number:39977-44-1
- Molecular formula:C8H9NO3
- Molecular Weight:167.16

Into a 100-mL round-bottom flask, was placed a solution of 2,6-dimethyl pyridine-2,6- dicarboxylate (950 mg, 4.87 mmol, 1.00 equiv) in a solvent mixture of methanol (33.2 mL) and dichloromethane (14.2 mL). NaB (185 mg, 5.02 mmol, 1.00 equiv) was added to the reaction mixture in several batches at 0°C. The resulting solution was stirred overnight at room temperature, and then it was quenched by the addition of 50 mL of NC1 (aq.). The resulting solution was extracted with 2x50 mL of dichloromethane and the combined organic layers were dried over anhydrous sodium sulfate and concentrated under vacuum. The residue was applied onto a silica gel column with ethyl acetate/petroleum ether (1 : 1-2: 1) as eluent to yield 750 mg (92%) of methyl 6-(hydroxymethyl)pyridine-2-carboxylate as a white solid.
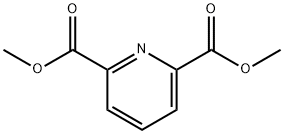
5453-67-8
298 suppliers
$6.00/5g

39977-44-1
154 suppliers
$13.00/250mg
Yield:39977-44-1 93%
Reaction Conditions:
with sodium tetrahydridoborate in methanol;dichloromethane at 0 - 20;
Steps:
Step-3: methyl 6-(hydroxymethyl)picolinate. (Xc)
Into a 500-ml round-bottom flask, was placed a solution of 2,6- dimethyl pyridine-2,6-dicarboxylate Xb (5g, 0.00487 mol) in a solvent mixture of methanol (174 ml) and dichloromethane (74 ml). NaBH4 (1.45 g, 0.00502 mol) was added to the reaction mixture in portions at 0°C. The resulting solution was stirred overnight at room temperature, and then it was quenched by the addition of Aq. NH4Cl (250 ml). The resulting solution was extracted with dichloromethane (2x200 ml) and the combined organic layers were dried over Na2SO4, filtered, and concentrated under vacuum. The residue was applied onto a silica gel column with ethyl acetate/petroleum ether (1:1) as eluent to yield methyl 6-(hydroxymethyl) pyridine-2-carboxylate Xc as white solid. Yield: (3.9 g, 93 %). LC_MS Calculated. for C8H9NO3 is 167.16; Observed.: 168.20 [M++1].1H NMR (400 MHz, CDCl3): δ 8.048-8.029 (d, J=7.6Hz, 1H), 7.875-7.837 (t, J=7.6Hz, 1H), 7.541-7.522 (d, J=7.6Hz, 1H), 4.863 (s, 2H), 4.002 (s, 3H).
References:
WO2022/149167,2022,A1 Location in patent:Paragraph 000123
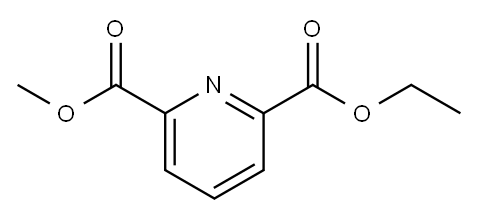
363593-99-1
0 suppliers
inquiry

39977-44-1
154 suppliers
$13.00/250mg
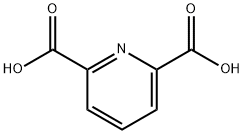
499-83-2
636 suppliers
$6.00/10g

39977-44-1
154 suppliers
$13.00/250mg

5453-67-8
298 suppliers
$6.00/5g
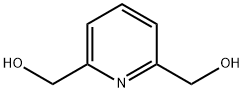
1195-59-1
381 suppliers
$6.00/1g

39977-44-1
154 suppliers
$13.00/250mg
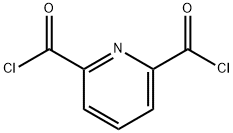
3739-94-4
138 suppliers
$5.00/1g

39977-44-1
154 suppliers
$13.00/250mg