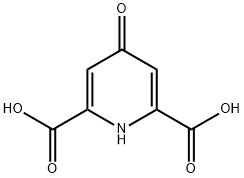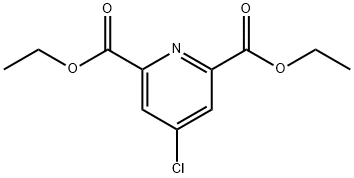
DIETHYL 4-CHLORO-2,6-PYRIDINEDICARBOXYLATE synthesis
- Product Name:DIETHYL 4-CHLORO-2,6-PYRIDINEDICARBOXYLATE
- CAS Number:53389-01-8
- Molecular formula:C11H12ClNO4
- Molecular Weight:257.67
71022-75-8

64-17-5

53389-01-8
The crude 4-chloropyridine-2,6-dicarbonyl dichloride was dissolved in dichloromethane (100 mL) under cooling conditions in an ice bath, followed by the slow addition of a mixture of pyridine (6 mL) and ethanol (5 mL). The reaction mixture was stirred in an ice bath for 5 minutes, then removed from the ice bath and continued to stir at room temperature for 5 hours. Upon completion of the reaction, the volatile solvent was removed by distillation under reduced pressure. The residue was washed with a mixture of 1 M hydrochloric acid (30 mL) and saturated aqueous sodium chloride solution (50 mL) and subsequently extracted with dichloromethane (200 mL). The organic phases were combined, dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by silica gel fast column chromatography using ethyl acetate-hexane (1:4, subsequently adjusted to 1:3) as eluent to give finally diethyl 4-chloro-2,6-pyridinedicarboxylate (800 mg, 62% yield). The product was characterized by 1H NMR (DMSO-d6): δ 1.36 (t, 6H, J = 7.1 Hz, CH3), 3.33 (s, 3H, CH3), 4.40 (q, 4H, J = 7.1 Hz, CH2), 8.31 (s, 2H, Ar).
Yield: 86%
Reaction Conditions:
Stage #1:Chelidamic acid with trichlorophosphateHeating / reflux;
Stage #2:ethanol with triethylamine in tetrahydrofuran at 0 - 20; for 0.5 h;
Steps:
34.A
Part A; Chelidamic acid 403 (5.00 g, 27.3 mmol) was mixed with 30 mL of POCI3 and heated to reflux overnight. The reaction was then concentrated in vacuo. The residue was dissolved in 50 mL of THF. This solution was added slowly to 200 mL of EtOH at 0 0C. Et3N (25 mL) was carefully added to the EtOH solution. The reaction was allowed to warm to room temperature and stirred for 30 minutes. This mixture was concentrated in vacuo and the residue dissolved in 200 mL of EtOAc. The EtOAc solution was washed with H2O and brine. The organic layer was dried with Na2SO4 and concentrated in vacuo. Recrystallization from EtOH gave 6.08 g of compound 404 in 86 % yields.
References:
SCHERING CORPORATION WO2008/82487, 2008, A2 Location in patent:Page/Page column 155
499-51-4
78 suppliers
$9.00/1g

53389-01-8
83 suppliers
$10.00/1g