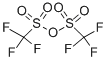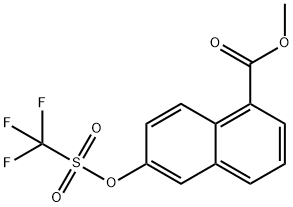
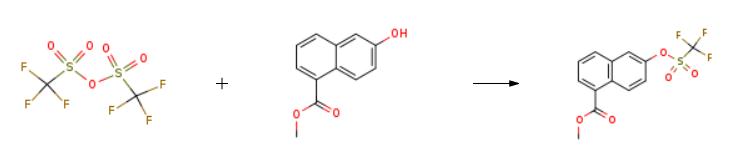
methyl 6-(trifluoromethylsulfonyloxy)-1-naphthoate synthesis
- Product Name:methyl 6-(trifluoromethylsulfonyloxy)-1-naphthoate
- CAS Number:255050-65-8
- Molecular formula:C13H9F3O5S
- Molecular Weight:334.27
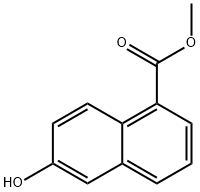
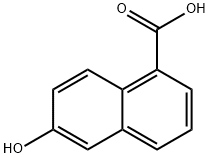
Diisopropylethyiamine (2.35 mL, 13,5 mmol) and irifluoromethanesuifonic anhydride (0,95 mL, 5.6 mmol) were added sequentially to a solution of methyl 6-hydroxy- l- naphthoate ( 10 mg, 4.5 mmol) in CH2C12 (60 mL) at - 78 °C. After 1 h at - 78 °C, the reaction mixture was poured into saturated aqueous NH4C1 (30 mL). The resulting mixture was extracted with CH2CI2 (3x50 mL). The combined organic phase was washed with brine (50 mL) then dried ( a2S0 ), filtered and concentrated in vacuo. The resulting residue was purified on 80 g silica gel (100% hexanes - 100% EtOAc, gradient) to afford 1 ,45 g (96%) of methyl 6-(trifluoromethylsulfonyloxy)-1-naphthoate.
Yield:255050-65-8 96%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at -78; for 1 h;
Steps:
1; 1.2
Diisopropylethyiamine (2.35 mL, 13,5 mmol) and irifluoromethanesuifonic anhydride (0,95 mL, 5.6 mmol) were added sequentially to a solution of methyl 6-hydroxy- l- naphthoate ( 10 mg, 4.5 mmol) in CH2C12 (60 mL) at - 78 °C. After 1 h at - 78 °C, the reaction mixture was poured into saturated aqueous NH4C1 (30 mL). The resulting mixture was extracted with CH2CI2 (3x50 mL). The combined organic phase was washed with brine (50 mL) then dried ( a2S0 ), filtered and concentrated in vacuo. The resulting residue was purified on 80 g silica gel (100% hexanes - 100% EtOAc, gradient) to afford 1 ,45 g (96%) of methyl 6-(((irifluoromethyl)s?lfony.)oxy)-l -naphthoate.
References:
WO2012/3145,2012,A2 Location in patent:Page/Page column 9; 20
90162-13-3
21 suppliers
inquiry

255050-65-8
5 suppliers
inquiry
2437-17-4
172 suppliers
$10.00/250mg

255050-65-8
5 suppliers
inquiry