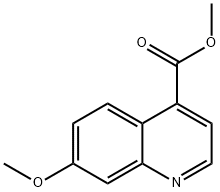
methyl 7-methoxyquinoline-4-carboxylate synthesis
- Product Name:methyl 7-methoxyquinoline-4-carboxylate
- CAS Number:1017969-31-1
- Molecular formula:C12H11NO3
- Molecular Weight:217.22

67-56-1
788 suppliers
$7.29/5ml-f
201230-82-2
1 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1017969-31-1
7 suppliers
inquiry
Yield: 57%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;triethylamine at 80; under 38002.6 Torr; for 16 h;Inert atmosphere;
Steps:
110.2; 111.2 [473] Step 2. Methyl 7-methoxyquinoline-4-carboxylate 2
[474] Into a 150-mL pressure tank reactor (90 atm), was placed 7-methoxyquinolin-4-yl trifluoromethanesulfonate (7.7 g, 25.06 mmol, 1.00 equiv), methanol (70 mL), triethylamine (12.67 g, 125.21 mmol, 5.00 equiv), Pd(dppf)Cl2 CH2C12 (4.09 g, 0.20 equiv), CO(gas. 50atm). The resulting solution was stirred for 16 h at 80 °C. The reaction was cooled to 20 °C. The solids were filtered out. The resulting mixture was concentrated under vacuum. The residue was applied onto a silica gel column with ethyl acetate/petroleum ether (1 :4). This resulted in 3.1 g (57%) of methyl 7-methoxyquinoline-4- carboxylate as off-white solid. MS (ES, m/z) [M+H]+: 218.
References:
THE ROCKEFELLER UNIVERSITY;PONDA, Manish, P.;BRESLOW, Jan, L.;SELNICK, Harold;EGBERTSON, Melissa WO2017/205296, 2017, A1 Location in patent:Paragraph 474

67-56-1
788 suppliers
$7.29/5ml-f
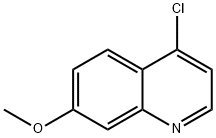
68500-37-8
200 suppliers
$18.00/250mg
201230-82-2
1 suppliers
inquiry

1017969-31-1
7 suppliers
inquiry
82121-05-9
184 suppliers
$58.00/5g

1017969-31-1
7 suppliers
inquiry