
Methyl cinnoline-6-carboxylate synthesis
- Product Name:Methyl cinnoline-6-carboxylate
- CAS Number:318276-74-3
- Molecular formula:C10H8N2O2
- Molecular Weight:188.18
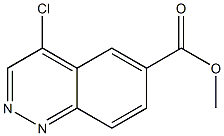
849805-63-6

318276-74-3
The general procedure for the synthesis of methyl cinnoline-6-carboxylate from methyl 4-chlorocinnoline-6-carboxylate was as follows: to a solution of methyl 4-chlorocinnoline-6-carboxylate (192 mg, 0.863 mmol) in dimethylsulfoxide (30 mL) was added sodium formate (70 mg, 1.04 mmol), tetrakis(triphenylphosphine)palladium(0) (198 mg, 0.702 mmol) and N,N-diisopropylethylamine (0.21 mL, 1.21 mmol). The reaction mixture was stirred at 90 °C for 1.5 hours. After completion of the reaction, it was cooled to room temperature, water was added and extracted with ethyl acetate. The organic layer was washed sequentially with water and brine, dried over anhydrous sodium sulfate and evaporated to remove the solvent. The resulting solid was washed with ether and subsequently purified by silica gel column chromatography (eluent: hexane-ethyl acetate) to afford methyl cinnoline-6-carboxylate (16 mg, 0.089 mmol, 10% yield). The product was characterized by 1H-NMR (CDCl3) with the following chemical shift δ (ppm): 4.03 (3H, s), 7.97 (1H, dd, J = 0.8, 6.0 Hz), 8.42 (1H, J = 0.8, 8.0 Hz), 8.59-8.63 (2H, m), and 9.43 (1H, dd, J = 0.8, 6.0 Hz).

67-56-1
790 suppliers
$9.00/25ml
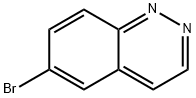
318276-72-1
80 suppliers
$45.00/10mg
201230-82-2
1 suppliers
inquiry

318276-74-3
37 suppliers
inquiry
Yield:318276-74-3 95.34%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;triethylamine at 60;Sealed tube;
Steps:
Preparation of Acid AE
Into a 30-mL sealed tube, was placed 6-bromocinnoline (600.00 mg, 2.870 mmol, 1.00 equiv), MeOH (12.00 L), Pd(dppf)Cl2(210.01 mg, 0.287 mmol, 0.10 equiv), TEA (871.30 mg, 8.611 mmol, 3.00 equiv), carbon monoxide (10 atm). The resulting solution was stirred for overnight at 60 degrees C in an oil bath. The resulting mixture was concentrated under vacuum. The resulting solution was diluted with 30 mL of H2O. The resulting solution was extracted with 3x20 mL of ethyl acetate and the organic layers combined and dried over anhydrous sodium sulfate and concentrated under vacuum. This resulted in 600 mg (95.34%) of methyl cinnoline-6- carboxylate as a red solid. LCMS (PH-PUK): [M+H]+=189.
References:
WO2021/127166,2021,A1 Location in patent:Paragraph 00135-00136

849805-63-6
4 suppliers
inquiry

318276-74-3
37 suppliers
inquiry
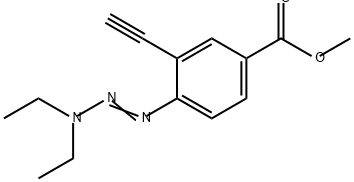
318276-66-3
0 suppliers
inquiry

318276-74-3
37 suppliers
inquiry
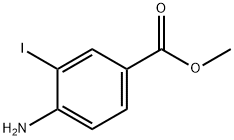
19718-49-1
122 suppliers
$8.00/1g

318276-74-3
37 suppliers
inquiry
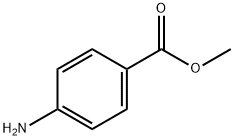
619-45-4
386 suppliers
$6.00/1g

318276-74-3
37 suppliers
inquiry