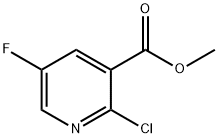
METHYL2-CHLORO-5-FLUORONICOTINATE synthesis
- Product Name:METHYL2-CHLORO-5-FLUORONICOTINATE
- CAS Number:847729-27-5
- Molecular formula:C7H5ClFNO2
- Molecular Weight:189.57
38186-88-8

18107-18-1

847729-27-5
Example 58 Synthesis of 4-((5-fluoro-2-(4-fluorophenyl)pyridin-3-yl]methyl}amino)methylbenzoic acid Procedure 1. Preparation of methyl 5-fluoro-2-(4-fluorophenyl)nicotinate: 2-chloro-5-fluoronicotinic acid (1.00 g, 5.70 mmol) was dissolved in a mixture of solvents of methanol (4 mL) and dichloromethane (14 mL) at 0 °C, and slowly added to a 2M hexane solution of trimethylsilylated diazomethane (5.70 mL, 11.4 mmol). The reaction mixture was stirred at 0 °C for 1 hour. Upon completion of the reaction, the reaction was quenched with acetic acid and the solvent was removed by concentration under reduced pressure. The residue was purified by fast column chromatography on silica gel using hexane/ethyl acetate (10:1, v/v) as eluent to afford 0.78 g (72% yield) of methyl 2-chloro-5-fluoronicotinate as a colorless oil. The product was characterized by 1H-NMR (CDCl3): δ 8.41 (1H, d, J=3.1 Hz), 7.93 (1H, dd, J=7.6,3.1 Hz), 3.98 (3H, s).

38186-88-8
242 suppliers
$6.00/1g

18107-18-1
210 suppliers
$33.00/5g

847729-27-5
87 suppliers
$12.00/100mg
Yield:847729-27-5 72%
Reaction Conditions:
in methanol;hexane;dichloromethane at 0; for 1 h;
Steps:
58.1
EXAMPLE 58 4- ( (F 5-FLUORO-2- (4-FLUOROBENZYL) PYRIDIN-3- YL1CARBONYL} AMINO) METHYL1BENZOIC ACID STEP 1. Methyl 5-FLUORO-2- (4-FLUOROBENZYL) nicotinate; 2-Chloro-5-fluoronicotinic acid (1.00 g, 5.70 mmol) was treated with 2 M solution of (Trimethylsilyl) DIAZOMETHANE in hexane (5.70 mL, 11.4 mmol), methanol (4 mL), and dichloromethane (14 mL) at 0 °C for 1 h. The mixture was quenched with acetic acid and concentrated under reduced pressure. The residue was purified by flush column chromatography on silica gel eluting with hexane/ethyl acetate (10/1) to afford 0.78 g (72%) of the title compounds as colorless oil : IH-NMR (CDC13) 6 8.41 (1H, d, J = 3. 1 HZ), 7.93 (1H, dd, J = 7. 6,3. 1 HZ), 3.98 (3H, s).
References:
PFIZER INC.;PFIZER JAPAN, INC. WO2005/21508, 2005, A1 Location in patent:Page/Page column 97

38186-88-8
242 suppliers
$6.00/1g

74-88-4
357 suppliers
$15.00/10g

847729-27-5
87 suppliers
$12.00/100mg

67-56-1
790 suppliers
$7.29/5ml-f

38186-88-8
242 suppliers
$6.00/1g

847729-27-5
87 suppliers
$12.00/100mg