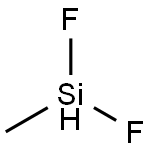
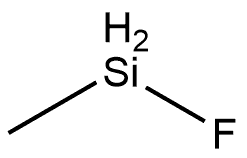
METHYLDIFLUOROSILANE synthesis
- Product Name:METHYLDIFLUOROSILANE
- CAS Number:420-34-8
- Molecular formula:CH4F2Si
- Molecular Weight:82.12
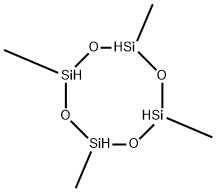
2370-88-9
280 suppliers
$17.00/5g

420-34-8
4 suppliers
inquiry
Yield: 77%
Reaction Conditions:
with boron trifluoride diethyl etherate in toluene at 60 - 90; for 0.416667 h;
Steps:
4 Example 4: Synthesis of difluoromethylsilane, DFMS, by reaction of 2,4,6,8- tetramethylcyclotetrasiloxane with BF3
Example 4: Synthesis of difluoromethylsilane, DFMS, by reaction of 2,4,6,8- tetramethylcyclotetrasiloxane with BF3
(0088) An apparatus and procedure as described in Example 1 was used for Example 4. Thus, 2,4,6, 8-tetramethylcyclotetrasiloxane 16.07 g (66.8 mmol) and toluene 100.06 g (1.09 mol) were charged to the four neck flask. Boron trifluoride diethyl etherate (BF3- OEt2) 26.24 g (184.9 mmol) was charged to the addition funnel. A dry ice/isopropanol slush bath was placed in the second addition funnel and in the bath under the two neck flask. BF3 - OEt2 was added drop- wise to the reaction flask over 25 minutes. No reflux or significant exotherm was observed. The reaction mixture was then heated initially to 60°C, whereupon refluxing commenced, and subsequently further heated to 90°C. The volatile product was collected in the second (two neck) flask and subsequently transferred to a storage cylinder. The collected product fraction was determined to be DFMS by NMR analysis: 519F = -138.50 ppm; δ = 4.85 ppm, 2J U-19F) = 69 Hz; δ Η^ = 0.47 ppm, 3J U-19F) = 6 Hz; = 3 Hz. The collected product (22.10 g) was 76 wt.% FDMS and thus the isolated yield was 77%
References:
ARKEMA INC.;SYVRET, Robert G.;POLSZ, Craig A. WO2016/32767, 2016, A1 Location in patent:Page/Page column 13
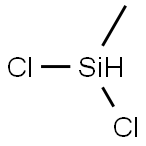
75-54-7
180 suppliers
$32.47/25G
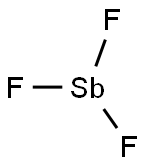
7783-56-4
157 suppliers
$23.12/5 g

420-34-8
4 suppliers
inquiry
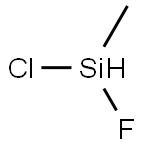
373-67-1
0 suppliers
inquiry
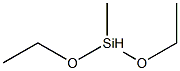
2031-62-1
179 suppliers
$28.00/5G

420-34-8
4 suppliers
inquiry
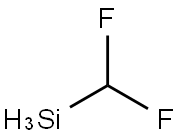
10112-10-4
0 suppliers
inquiry
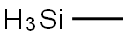
992-94-9
0 suppliers
$932.00/10g
753-44-6
0 suppliers
inquiry

420-34-8
4 suppliers
inquiry
373-74-0
36 suppliers
inquiry