
N-(1H-Indol-5-Ylmethyl)-N-Methylamine synthesis
- Product Name:N-(1H-Indol-5-Ylmethyl)-N-Methylamine
- CAS Number:709649-72-9
- Molecular formula:C10H12N2
- Molecular Weight:160.22
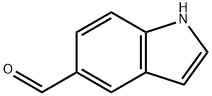
1196-69-6
311 suppliers
$9.00/1g

74-89-5
0 suppliers
$13.44/25ML

709649-72-9
23 suppliers
$285.31/250mg
Yield:709649-72-9 91%
Reaction Conditions:
Stage #1: 1H-indole-5-carboxaldehyde;methylamine in methanol at 25; for 0.5 h;
Stage #2: with methanol;sodium tris(acetoxy)borohydride at 25; for 3 h;
Steps:
Step 1
To a stirred solution of 1H-indole-5-carbaldehyde (1.00 g, 6.89 mmol) in MeOH (10.0 mL) was added MeNH2(1.43 g, 30%wt. in MeOH, 13.8 mmol) at 25 . The resultant mixture was stirred at that temperature for 30 min before NaBH (OAc)3(1.59 g, 7.50 mmol) was added. The reaction mixture was stirred at 25 for 3 h before it was quenched with saturated aq. NaHCO3(20 mL) . The resultant mixture was extracted with EtOAc (50 mL × 3) . The combined organic phases were washed with brine (50 mL) , dried over anhydrous Na2SO4, and filtered. The solvent was evaporated under vacuum to give 1- (1H-indol-5-yl) -N-methylmethanamine (1.01 g, 91%yield) as a brown solid which was used in the next step without further purification. LC-MS: m/z [M+H]+161.0.
References:
WO2022/105930,2022,A1 Location in patent:Page/Page column 65

1154383-44-4
4 suppliers
inquiry

709649-72-9
23 suppliers
$285.31/250mg

1196-69-6
311 suppliers
$9.00/1g

709649-72-9
23 suppliers
$285.31/250mg