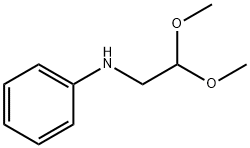
N-(2,2-dimethoxyethyl)aniline synthesis
- Product Name:N-(2,2-dimethoxyethyl)aniline
- CAS Number:26972-56-5
- Molecular formula:C10H15NO2
- Molecular Weight:181.23
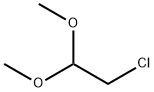
97-97-2
299 suppliers
$24.00/25mL

62-53-3
688 suppliers
$10.00/1g

26972-56-5
17 suppliers
$45.00/10mg
Yield:26972-56-5 49%
Reaction Conditions:
Stage #1: anilinewith sodium hydride in tetrahydrofuran; for 0.5 h;
Stage #2: chloroacetaldehyde dimethyl acetal in tetrahydrofuran at 20;
Steps:
1.1.3Synthesis of aminoacetaldehyde dimethyl acetals 2o-2q
General procedure: 0.6 g of sodium hydride (NaH) powder was dissolved 20 mL of dry THF in a round bottom flask. To that mixture was added an aniline derivative (60 mmol) solution in dry THF (10 mL) drop by drop. After stirring for 30 min, Chloroacetaldehyde dimethyl acetal (2.49 g, 2.26 mL,20 mmol) in dry THF (10 mL) was dropwisely added. The reaction was continued to stirred at room temperature and monitored by TLC. the reaction was then diluted slowly with water and extracted with ether (3x50 mL). The organic layer was combined and dried over MgSO4 anhydrous. The solvent was removed under reduced pressure and the residue was purified by column chromatography (SiO2; 4:1 hexanes/ethyl acetate).
References:
Luu, Quang H.;Guerra, Jorge D.;Casta?eda, Cecilio M.;Martinez, Manuel A.;Saunders, Jong;Garcia, Benjamin A.;Gonzales, Brenda V.;Aidunuthula, Anushritha R.;Mito, Shizue [Tetrahedron Letters,2016,vol. 57,# 21,p. 2253 - 2256] Location in patent:supporting information

62-53-3
688 suppliers
$10.00/1g

26972-56-5
17 suppliers
$45.00/10mg