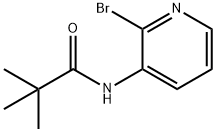
N-(2-Bromopyridin-3-yl)pivalamide synthesis
- Product Name:N-(2-Bromopyridin-3-yl)pivalamide
- CAS Number:835882-02-5
- Molecular formula:C10H13BrN2O
- Molecular Weight:257.13
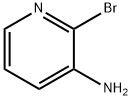
39856-58-1
305 suppliers
$10.00/1g
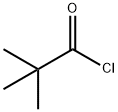
3282-30-2
358 suppliers
$19.85/100ml

835882-02-5
36 suppliers
$34.00/100mg
Yield:835882-02-5 93%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 3 h;
Steps:
4.1 N-(2-bromopyridin-3-yl)pivalamide (X)
To a solution of 1 .13 g (6.5 mmol, 1 eq.) of 3-amino-2-bromopyridine and 1 .2 mL (8.5 mmol, 1 .3 eq.) of triethylamine in 13 mL of DCM, cooled to 0°C, were added 0.9 mL (7.2 mmol, 1 .1 eq.) of trimethylacetyl chloride in 2 mL of DCM. After 3 hours at room temperature, 15 mL of water were added. The aqueous layer was extracted with 2 x 10 mL of dichloromethane. The organic phase was dried, filtered and concentrated. Purification over silica gel provided 1 .59 g (6.1 mmol, 93%) of X as a colourless oil. Rf=0.68 (PE/EtOAc 2: 1 ). 1 H NMR (300 MHz): δ = 8.69 (dd, J = 8.1 Hz, J = 1 .6 Hz, 1 H), 8.07 (dd, J = 4.6 Hz, J = 1 .6 Hz, 1 H), 8.02 (broad, 1 H), 7.26 (dd, J = 8.1 Hz, J = 4.7 Hz, 1 H), 1 .35 (s, 9 H) ppm. 13C NMR (75 MHz): δ = Ml A, 144.4, 133.8, 133.5, 128.6, 123.7, 40.3, 27.6 ppm
References:
WO2014/198844,2014,A1 Location in patent:Page/Page column 19