
N-(2-CYANOPHENYL)-N''-PHENYLUREA synthesis
- Product Name:N-(2-CYANOPHENYL)-N''-PHENYLUREA
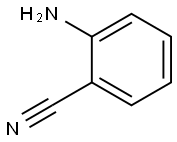
- CAS Number:13114-96-0
- Molecular formula:C14H11N3O
- Molecular Weight:237.26
Yield:13114-96-0 89%
Reaction Conditions:
with dimethyl sulfoxide;2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide at 20; for 4 h;Green chemistry;Temperature;Time;
Steps:
General Procedure for the synthesis of o-ureidobenzonitriles (3a-3t)
General procedure: The mixture of o-aminobenzamide (1) (1 mmol) and isothiocyanates (2) (1 mmol) was allowed to stir in DMSO solvent and T3P (Propane phosphonic acid anhydride solution: 50 wt. % in ethyl acetate) reagent (1 mmol, 0.7 g) at room temperature for 4 h in a round bottomed flask. After that, the contents of the flask were poured in to 15 ml of water taken in separating funnel and extracted to 15 ml ethyl acetate. The ethyl acetate extract was separated and dried over anhydrous sodium sulfate. The solvent was then removed under reduced pressure to afford corresponding o-ureidobenzonitrile (3). The crude residue thus obtained was purified by column chromatography over silica gel using 10 % ethyl acetate in hexane as eluent to afford the pure o-ureidobenzonitrile with 80-95% yields.
References:
Shamanth, Sadashivamurthy;Nagarakere, Sandhya C.;Sagar, Kunigal S.;Narayana, Yatheesh;Mamatha, Mahesha;Rangappa, Kanchugarakoppal S.;Kempegowda, Mantelingu [Synthetic Communications,2021,vol. 51,# 8,p. 1197 - 1205] Location in patent:supporting information
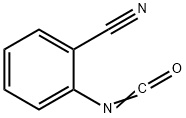
42066-86-4
69 suppliers
$75.00/1g

62-53-3
679 suppliers
$10.00/1g

13114-96-0
16 suppliers
$28.60/50mg

73859-66-2
0 suppliers
inquiry

13114-96-0
16 suppliers
$28.60/50mg
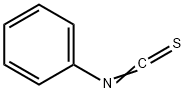
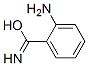
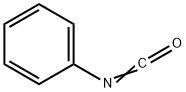
![1H-Indole-2,3-dione, 3-[O-(phenylsulfonyl)oxime]](/CAS/20210305/GIF/749250-84-8.gif)