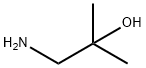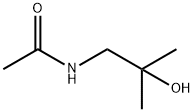
N-(2-hydroxy-2-methylpropyl)acetamide synthesis
- Product Name:N-(2-hydroxy-2-methylpropyl)acetamide
- CAS Number:37150-62-2
- Molecular formula:C6H13NO2
- Molecular Weight:131.17
Yield:37150-62-2 57%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;chlorotri(pyrrolidin-1-yl)phosphonium hexafluorophosphate in N,N-dimethyl-formamide at 0 - 20; for 12 h;
Steps:
3.2. General Procedures for the Synthesis of N-Alkyl Amides 2a-k, 3a-k, and 4a-k
General procedure: N-alkyl amides 2a-k, 3a-k, and 4a-k were synthesized using the method reported in the previousreference. The PyClOP (10 mmol) was added to a solution of carboxylic acid 1a-k (10 mmol),amine (butylamine, 2-methyl allylamine, or 1-amino-2-methyl propan-2-ol, 10 mmol), and DIEA(20 mmol) in 30 mL of dimethylformamide (DMF) at 0 °C. Next, the mixture solution was continuouslystirred for 12 h at room temperature and then diluted with 20 mL water and 20 mL ethyl acetate.The organic layer was washed with 100 mL brine twice, 100 mL dilute HCl (1 mol/L) twice, 100 mLwater twice, 100 mL 5% NaHCO3 twice, and 100 mL brine twice. The resulting mixture solution wasconcentrated under vacuum and further purified by flash chromatography on a silica gel column(ethyl acetate: petroleum ether, 1:6) to aord the N-alkyl amides 2a-k, 3a-k, and 4a-k (yields 34-84%)as colorless oils, yellow oils, white solids, or yellow solids. 1H NMR and 13C NMR spectrums of targetcompounds 2a-k, 3a-k, and 4a-k see the Supplementary Materials.
References:
Deng, Xile;Zheng, Wenna;Zhan, Qingcai;Deng, Yanan;Zhou, Yong;Bai, Lianyang [Molecules,2020,vol. 25,# 21,art. no. 4986]