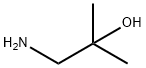
1-Amino-2-methylpropan-2-ol synthesis
- Product Name:1-Amino-2-methylpropan-2-ol
- CAS Number:2854-16-2
- Molecular formula:C4H11NO
- Molecular Weight:89.14
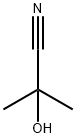
75-86-5

2854-16-2
The general procedure for the synthesis of 1-amino-2-methyl-2-propanol from acetone cyanohydrin is as follows: Step A: Synthesis of 1-amino-2-methyl-2-propanol [Chem.17] To a tetrahydrofuran (180 mL) suspension of lithium aluminum hydride (6.08 g, 160 mmol), a tetrahydrofuran (20 mL) solution of acetone cyanohydrin (7.32 mL, 80.0 mmol) was slowly added dropwise over a controlled period of 15 min at 0 °C. The reaction mixture was then refluxed for 4 hours. Upon completion of the reaction, the mixture was cooled to 0 °C and the reaction was quenched with sodium sulfate decahydrate and potassium fluoride. After continued stirring at 35°C for 30 minutes, the mixture was filtered through a diatomaceous earth pad. The filtrate was concentrated in vacuum to give the oily product 1-amino-2-methyl-2-propanol (3.98 g, 56% yield). [0229] 1H-NMR (300 MHz, CDCl3) δ: 2.60 (s, 2H), 1.69 (s, 6H), no peaks for OH and NH2 were observed.
625-08-1
240 suppliers
$18.00/5g

2854-16-2
234 suppliers
$14.00/1g
Yield:2854-16-2 85%
Reaction Conditions:
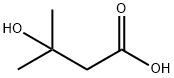
Stage #1: 3-Hydroxy-3-methylbutyric acidwith 4-methyl-morpholine;diphenyl phosphoryl azide in tetrahydrofuran at 25; for 2.25 h;Curtius Rearrangement;
Stage #2: with water in tetrahydrofuran; for 2 h;Reflux;Reagent/catalyst;
Steps:
2.1 (1) Synthesis of 2-methyl-2-hydroxypropylamine (Intermediate 6)
5.91 g (50 mmol) of [beta] -hydroxyisovaleric acid was dissolved in 150 mL of THF,After adding 15 mL of N-methylmorpholine, 13.76 g (50 mmol) of diphenyl azide was added dropwise at room temperature,After 15 min dripping, the reaction was stirred for 4 h.Then add 60 mL of distilled water, stir and reflux for 2 h.After the reaction, the THF was evaporated under reduced pressure.Add 30 mL of ethyl acetate, stir,Adjust the pH to 2-3. The two phases were separated and the aqueous phase was extracted twice with ethyl acetate twice. The aqueous phase was further added 30 mL of ethyl acetate,Adjust the pH to 9 or so. Separate the two phases, extracted twice with water, 15 mL each time. Dried Na2SO4 and evaporated to dryness to obtain 3.79 gProduct (yield 85%).
References:
CN106496085,2017,A Location in patent:Paragraph 0018; 0019; 0023
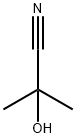
75-86-5
0 suppliers
$64.90/5G

2854-16-2
234 suppliers
$14.00/1g
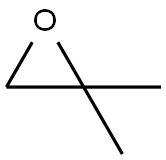
558-30-5
246 suppliers
$20.00/5G

2854-16-2
234 suppliers
$14.00/1g
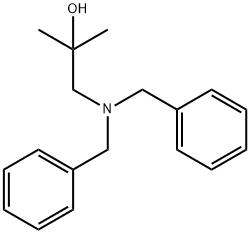
344868-41-3
0 suppliers
inquiry

2854-16-2
234 suppliers
$14.00/1g
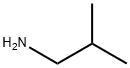
78-81-9
218 suppliers
$19.60/250ml

2854-16-2
234 suppliers
$14.00/1g