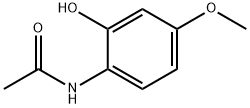
N-(2-Hydroxy-4-Methoxyphenyl)acetaMide synthesis
- Product Name:N-(2-Hydroxy-4-Methoxyphenyl)acetaMide
- CAS Number:58469-06-0
- Molecular formula:C9H11NO3
- Molecular Weight:181.19
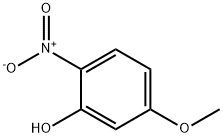
704-14-3
141 suppliers
$7.00/1g

108-24-7
0 suppliers
$14.00/250ML

58469-06-0
39 suppliers
$134.00/50MG
Yield:58469-06-0 80%
Reaction Conditions:
Stage #1: 3-methoxy-6-nitro-phenolwith hydrogen;palladium 10% on activated carbon in tetrahydrofuran at 20;
Stage #2: acetic anhydride in tetrahydrofuran;water at 60; for 1 h;
Steps:
3.I Example 3; N-(2-{[(2S)-3-(5-chloro-1'H,3H-spiro[1-benzofuran-2,4'-piperidin]-1'-yl)-2-hydroxypropyl]oxy}-4-methoxyphenyl)acetamide; Step I:; N-(2-hydroxy-4-methoxyphenyl)acetamide
2-nitro-5-methoxyphenol (prepared from 3-methoxyphenol, R. J. Maleski, Synthetic Communications, 1993, 23, 343-348) (48.5g, 0.287 mol) dissolved in THF (1.5 L) was hydrogenated at ambient temperature over night with 10% palladium on carbon (10 g) until 20.3 L of hydrogen was consumed. After filtration and evaporation the residue was suspended in degased water (1.7 L) and acetic anhydride (42.5 mL) was added with stirring. The mixture was heated to 60°C for 1 h and then cooled to room temperature. The volatiles were removed in vacuo and the solid was washed thoroughly with water and dried in vacuo to give brick-red crystals (41.7 g, 80 %). 1H-NMR (400 MHz, CDCl3) : δ 8.98 (s, 1H) ; 7.34 (br. s, 1H); 6,81 (d, 1H) ; 6. 58 (d, 1H) ; 6.44 (dd, 1H) ; 3.78 (s, 3H); 2,26 (s, 3H)
References:
WO2004/5295,2004,A1 Location in patent:Page 72-73
51-66-1
231 suppliers
$12.00/1mg

58469-06-0
39 suppliers
$134.00/50MG
40925-70-0
77 suppliers
$32.00/100mg

108-24-7
0 suppliers
$14.00/250ML

58469-06-0
39 suppliers
$134.00/50MG
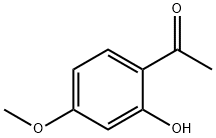
552-41-0
609 suppliers
$5.00/1g

58469-06-0
39 suppliers
$134.00/50MG
