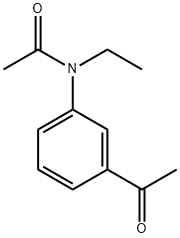
N-(3-Acetylphenyl)-N-ethylacetaMide synthesis
- Product Name:N-(3-Acetylphenyl)-N-ethylacetaMide
- CAS Number:200630-96-2
- Molecular formula:C12H15NO2
- Molecular Weight:205.25
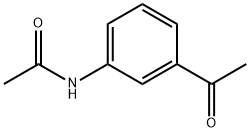
7463-31-2
204 suppliers
$12.00/5g

75-03-6
369 suppliers
$11.00/5g

200630-96-2
14 suppliers
$650.00/250mg
Yield:200630-96-2 100%
Reaction Conditions:
with potassium hydroxide in acetone at 20;
Steps:
3
Acetamidoacetophenone (0. 2 g, 1. 13 mmol) in ME2CO (2 mL) was treated with KOH (63 mg, 1. 13 mmol) and then iodoethane (0. 45 mL, 5. 64 mmol). After stirring at room temperature overnight the reaction mixture was concentrated to dryness. The residue was redissolved in EtOAc and was washed with H20 and brine, and was dried on MGS04. The solvent was evaporated to yield N-(3-acetyl-phenyl)-N-ethyl-acetamide as an orange powder (0. 23 g, 100 %) : mp 203-204 °C ; 1H-NMR (CD30D) : No. 1. 11 (t, 3H, J= 7. 0 Hz, CH3), 1. 82 (s, 3H, CH3), 3. 31 (s, 3H, CH3), 3. 77 (q, 2H, J = 7. 0, 14. 0 Hz, CH2) 7. 56 (d, 1H, J=8. 0 Hz, Ph-H), 7. 65 (t, 1H, J= 8 Hz, Ph-H), 7. 88 (s, 1H, Ph-H) and 8. 06 (d, 1H, J = 8 Hz, Ph-H) ; MS (ESF) m/z 205. 91 [M], C12H15NO2 requires 205. 25. This material (0. 23G, 1. 13 mmol), redissolved in MECN (2 mL), was treated with N,N- dimethylformamide dimethylacetal (150 UL, 1. 12 mmol) at 180 °C FOR 10 min in a microwave reactor (SmithCreator, Personal Chemistry Ltd.). The solvent was evaporated and the residue was filtered and washed with EtOAc/PE (1 : 3) to afford N [3- (3- DIMETHYLAMINO-ACRYLOYL)-PHENYL]-N-ETHYL-ACETAMIDE as an orange solid (0. 30 g, 100 %). 1H-NMR (CD30D) : 61. 11 (t, 3H, J= 7. 0 Hz, CH3), 1. 82 (s, 3H, CH3), 2. 04 (s, 6H, CH3), 3. 76 (q, 2H, J= 7. 0, 14. 0 Hz, CH2), 5. 87 (d, 1H, J= 12. 0 Hz, CH), 7. 39 (d, 1H, J= 8. 0 Hz, Ph-H), 7. 55 (t, 1H, J= 8. 0 Hz, 5-H), 7. 76 (s, 1H, Ph-H), 7. 89 (d, 1H, J= 12. 0 Hz, CH), 7. 93 (d, 1H, J = 8. 0 Hz, Ph-H) ; MS (ESI+) m/z 261. 32 [M+H]+, C15H20N2O2 requires 260. 33. A solution of this material (0. 228 g, 0. 88 mmol), 4-hydroxy-phenyl guanidine nitrate (0. 188 g, 0. 88 mmol) and NAOH (35 mg, 0. 88 mmol) in MeCN (2 mL) was heated at 190 °C for 15 min in the microwave reactor. The solvent was evaporated and the residue was purified by Si02 gel chromatography (EtOAc/PE, 1 : 1) to afford the title compound as a yellow solid (117 mg, 38 %). IH-NMR (CD30D) : No.1. 16 (t, 3H, J= 7 Hz, CH3), 3. 35 (s, 3H, CH3), 3. 38 (q, 2H, J= 7. 0, 14. 0 Hz, CH2), 6. 77 (d, 2H, J= 9. 0 Hz, Ph-H), 7. 27 (d, 1H, J= 5. 0 Hz, pyrimidine-H), 7. 42 (d, 1H, J= 8. 0 Hz, Ph-H), 7. 48 (d, 2H, J= 9. 0 Hz, Ph-H), 7. 62 (t, 1H, J= 8. 0 Hz, Ph-H), 8. 6 (s, 1H, Ph-H), 8. 14 (d, 1H, J=8. 0 Hz, Ph-H), 8. 41 (d, 1H, J= 5. 0 Hz, pyrimidine-H).
References:
WO2005/12262,2005,A1 Location in patent:Page/Page column 45-46
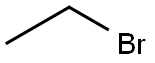
74-96-4
442 suppliers
$9.00/5g

7463-31-2
204 suppliers
$12.00/5g

200630-96-2
14 suppliers
$650.00/250mg
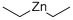
557-20-0
230 suppliers
$14.00/1g

75-36-5
589 suppliers
$17.92/100G
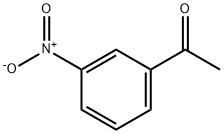
121-89-1
422 suppliers
$5.00/10g

200630-96-2
14 suppliers
$650.00/250mg
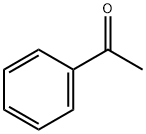
98-86-2
794 suppliers
$9.00/5g

200630-96-2
14 suppliers
$650.00/250mg

121-89-1
422 suppliers
$5.00/10g

200630-96-2
14 suppliers
$650.00/250mg