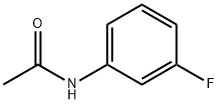
N-(3-fluoro-4-nitrophenyl)acetamide synthesis
- Product Name:N-(3-fluoro-4-nitrophenyl)acetamide
- CAS Number:345-30-2
- Molecular formula:C8H7FN2O3
- Molecular Weight:198.15
Yield:345-30-2 91%
Reaction Conditions:
at 22; for 16 h;Inert atmosphere;
Steps:
47 Preparation 47: N-(3-fluoro-4-nitrophenyl)acetamide
3-Fluoro-4-nitroaniline (20 g, 128.1 mmol) was treated with Ac2O (250 ml_). The mixture was stirred for 16 h at RT and then diluted with water (100 ml_). The precipitate was collected by filtration. The solids were taken up in EtOAc (100 ml_), dried (Na2SO4), filtered and concentrated to give the title compound 47 (23.0 g, 91% yield).1H NMR(400 MHz, CD3OD) d 8.09 (t, 1 H), 7.86 (dd, 1 H), 7.38 (dt, 1 H), 2.17 (s, 3H).
References:
WO2021/124155,2021,A1 Location in patent:Page/Page column 53; 76
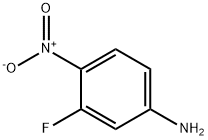
2369-13-3
249 suppliers
$19.00/5g

345-30-2
25 suppliers
inquiry
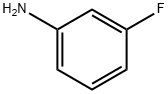
372-19-0
320 suppliers
$10.00/1g

345-30-2
25 suppliers
inquiry