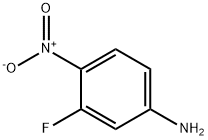
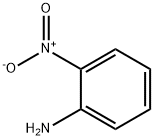
3-Fluoro-4-nitroaniline synthesis
- Product Name:3-Fluoro-4-nitroaniline
- CAS Number:2369-13-3
- Molecular formula:C6H5FN2O2
- Molecular Weight:156.11
372-19-0

2369-13-3
The general procedure for the synthesis of 3-fluoro-4-nitroaniline using 3-fluoroaniline as starting material is as follows: 1. 58 g of 3-fluoroaniline was reacted with 58 mL of benzaldehyde by heating at 80 °C for 1 hour. Subsequently, 200 mL of sulfuric acid was added to the reaction mixture and cooled with an ice bath. After removing the ice bath, stirring was continued at room temperature until the solid was completely dissolved. 2. The reaction mixture was again cooled to 0°C with an ice bath, and a mixture consisting of 36 mL of nitric acid and 120 mL of sulfuric acid was added slowly and dropwise, keeping the reaction temperature at 0°C. After stirring at 0 °C for 1 h, the solid was collected by filtration and poured into saturated aqueous potassium carbonate solution. 3. Ethyl acetate was added to the mixture and the organic and aqueous layers were separated. The aqueous phase was extracted twice with ethyl acetate. The organic phases were combined, dried with anhydrous magnesium sulfate and concentrated. The crude product was purified by silica gel column chromatography using a hexane solution of 40% ethyl acetate as eluent to give 28.65 g (35% yield) of Intermediate A-2 (3-fluoro-4-nitroaniline). 4. 28.65 g of Intermediate A-2 was dissolved in 236 mL of 36% hydrochloric acid and cooled to 0 °C in an ice bath. 13.7 g of sodium nitrite was added in batches and the reaction was kept at 0 °C for 1.5 hours. Subsequently, the reaction mixture was mixed with 145 mL of SO2 saturated acetic acid solution containing 10.5 mL of water and 9.3 g of CuCl2-2H2O. 5. The cooling bath was removed and the reaction mixture was stirred at room temperature for 1 h before being poured into ice water. The solid was collected by filtration to give 37.7 g of Intermediate B-2 (3-fluoro-4-nitrobenzenesulfonyl chloride). 6. In 500 mL of tetrahydrofuran, 53 g of Intermediate C-1 (PG = Boc, R4 = isobutyl) was mixed with 42 mL of triethylamine, and 37.7 g of Intermediate B-2 was added in one portion. the reaction mixture was stirred overnight at room temperature and concentrated. The residue was dissolved in ethyl acetate and washed sequentially with water, 5% aqueous hydrochloric acid and aqueous potassium carbonate. The organic layer was dried over anhydrous magnesium sulfate and concentrated. The crude product was purified by silica gel column chromatography to give 53 g (65% yield) of Intermediate 2-a. 7. 2 g of Intermediate 2-a was dissolved in 50 mL of N,N-dimethylformamide and 1.85 mL of isopropylamine was added. the reaction mixture was stirred at 60 °C overnight and concentrated. The residue was treated with a mixture of ethyl acetate and saturated saline, and the organic layer was dried over anhydrous magnesium sulfate and concentrated to give 2 g (91% yield) of Intermediate 2-b, which was used directly in the next step of the reaction. 8. 2 g of Intermediate 2-b was dissolved in 40 mL of methanol, 1.5 g of ammonium formate and 0.2 g of 10% palladium carbon were added. the reaction mixture was stirred overnight at 60 °C and supplemented with 0.5 g of ammonium formate and 0.2 g of palladium carbon, and the reaction was continued for 3 hours. The reaction mixture was filtered through diatomaceous earth and concentrated. The residue was dissolved in 50 mL of dichloromethane and washed sequentially with aqueous sodium carbonate and saturated brine, and the organic layer was dried over anhydrous magnesium sulfate and concentrated to give 1.3 g (68% yield) of Intermediate 2-c without further purification. 9. 1.3 g of Intermediate 2-c was dissolved in 20 mL of triethyl orthoformate, and the reaction was stirred at 80 °C for 5 h and then concentrated. The residue was dissolved in ethyl acetate and washed with aqueous sodium carbonate. The organic layer was dried with anhydrous magnesium sulfate and concentrated. The crude product was purified by silica gel column chromatography using a dichloromethane solution of 0 to 2% methanol as eluent to give 0.8 g (70% yield) of Intermediate 2-d. 10. 2.6 g of Intermediate 2-d was dissolved in 100 mL of isopropanol solution of 5N hydrochloric acid and concentrated after stirring the reaction for 2 hours at room temperature to give 2.5 g (94% yield) of deprotected amine hydrochloride 2-e. 11. 2.5 g of Intermediate 2-e and 1.5 mL of triethylamine were dissolved in 60 mL of dichloromethane and 3.05 g of 1-[[(3R,3aR,6aR)-hexahydrofuro[2,3-b]furan-3-yl]oxycarbonyloxy]-2,5-pyrrolidinedione was added. After stirring the reaction mixture for 4 hours at room temperature, it was washed with aqueous sodium carbonate.
1152311-98-2
1 suppliers
inquiry

2369-13-3
249 suppliers
$19.00/5g
Yield:2369-13-3 99%
Reaction Conditions:
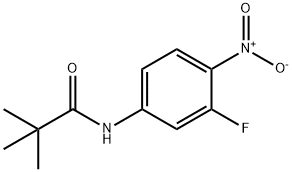
Stage #1:N-(3-fluoro-4-nitro-phenyl)-2,2-dimethyl-propionamide with hydrogenchloride in dichloromethane;water for 2 h;Reflux;
Stage #2: with potassium carbonate in dichloromethane;water;ethyl acetate
Steps:
9.a
A mixture of N-(3-fluoro-4-nitro-phenyl)-2, 2-dimethyl-propionamide (87.0 g, 0.36 mol) in CH2Cl2 (400 mL) and 6N hydrochloric acid (800 mL) was heated to reflux for 2 hours. The reaction mixture was cooled to room temperature. The reaction mixture was diluted with 1000 mL of ethyl acetate and potassium carbonate (500.0 g) was added portion wise. The aqueous solution was separated and the organic layer was washed with brine and dried over anhydrous Na2SO4. The solvent was removed by evaporation under reduced pressure; the residue was purified by column chromatography on silica gel (petroleum ether / ethyl acetate 30: 1) to afford 3-fluoro-4-nitroaniline (56.0 g, 99 %). 1H NMR (300 MHz, CDCl3) δ 8.07 (t, J = 8.7 Hz, 1 H), 7.86 (dd, J= 2.1, 13.2 Hz 1 H), 7.59 (brs, 2 H), 7.22 (s, 1 H).
References:
VERTEX PHARMACEUTICALS INCORPORATED;RUAH, Sara, Hadida, S.;GROOTENHUIS, Peter, D., J.;VAN GOOR, Fredrick;MILLER, Mark, T.;MCCARTNEY, Jason;ZHOU, Jinglan;BEAR, Brian;NUMA, Mehdi, Michel, Djamel WO2010/53471, 2010, A1 Location in patent:Page/Page column 56

372-19-0
320 suppliers
$10.00/1g

2369-13-3
249 suppliers
$19.00/5g
403-21-4
258 suppliers
$6.00/1g

75-65-0
784 suppliers
$10.00/10ml
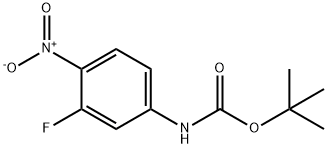
658700-15-3
11 suppliers
inquiry

2369-13-3
249 suppliers
$19.00/5g

658700-15-3
11 suppliers
inquiry

2369-13-3
249 suppliers
$19.00/5g
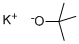
865-47-4
715 suppliers
$14.00/25g
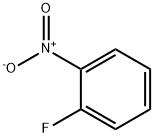
1493-27-2
510 suppliers
$5.00/10g
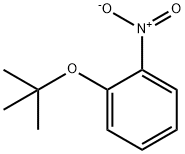
83747-12-0
14 suppliers
inquiry

2369-13-3
249 suppliers
$19.00/5g
88-74-4
59 suppliers
$10.00/1g