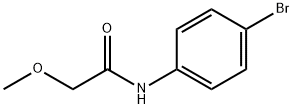
N-(4-bromophenyl)-2-methoxyacetamide synthesis
- Product Name:N-(4-bromophenyl)-2-methoxyacetamide
- CAS Number:104703-38-0
- Molecular formula:C9H10BrNO2
- Molecular Weight:244.09
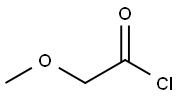
38870-89-2
233 suppliers
$22.00/5g

106-40-1
483 suppliers
$12.00/5g

104703-38-0
8 suppliers
$31.00/250mg
Yield:-
Reaction Conditions:
with pyridine in tetrahydrofuran at 0;Inert atmosphere;
Steps:
48 Example 48
Compound 129 (1000mg, 5.81mmol) was dissolved in THF (10 ml), then pyridine (0.938mL, 11.63mmol) was added thereto. The reaction mixture was cooled to 0°C under nitrogen atmosphere. Compound 142 (0.584mL, 6.39mmol) was added thereto and the mixture was stirred at 0°C. Water and a 2 mol/L aqueous solution of hydrochloric acid were added thereto, and the mixture was extracted with ethyl acetate. The organic layer was washed with water and an aqueous solution of hydrochloric acid. The obtained organic layer dried over magnesium sulfate, filtered, and concentrated under reduced pressure. Hexane was added to the obtained residue and Compound 143 was obtained by filtration. Compound (I-2-10) was synthesized from Compound 55 and Compound 143, in a similar way that Compound (I-2-05) was synthesized from Compound 55 and Compound 131. Compound (I-2-10); Method B LC/MS retention time = 1.19 min. MS (ESI) m/z = 520.15(M+H)+.
References:
EP3421467,2019,A1 Location in patent:Paragraph 0323-0324
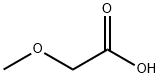
625-45-6
322 suppliers
$17.00/25g

104703-38-0
8 suppliers
$31.00/250mg

104703-42-6
0 suppliers
inquiry

104703-38-0
8 suppliers
$31.00/250mg