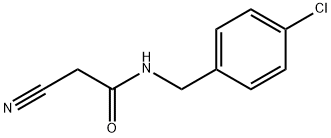
N-(4-CHLOROBENZYL)-2-CYANOACETAMIDE synthesis
- Product Name:N-(4-CHLOROBENZYL)-2-CYANOACETAMIDE
- CAS Number:66158-49-4
- Molecular formula:C10H9ClN2O
- Molecular Weight:208.64
Yield:66158-49-4 89%
Reaction Conditions:
with benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in 1,4-dioxane at 20; for 3 h;
Steps:
1.1(A)
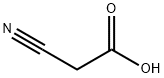
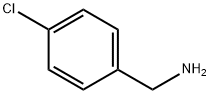
According to the Scheme 1 Step 1: A mixture of 4-chlorobenzylamine (10.0 g, 71 mmol), cyanoacetic acid (6.13 g, 72 mmol), HOBt (11 g, 72 mmol), EDCI.HCl (20 g, 106 mmol) in 100 mL of dioxane was stirred for 3 h at room temperature. The solvent was removed under vacuum and 300 mL of DCM was added. The organic layer was successively washed with 2×100 mL of water, 2×50 mL of a solution 1N of NaOH, 4×100 mL of water until neutral pH. The organic layer was dried over Na2SO4, filtered and concentrated to afford N-(4-chlorobenzyl)-2-cyanoacetamide 1(A) (13.12 g, 89%) as a brown solid.
References:
US2010/4246,2010,A1 Location in patent:Page/Page column 26