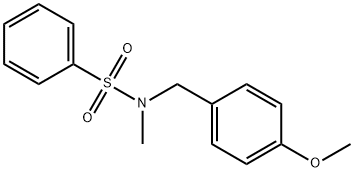
N-(4-METHOXYBENZYL)-N-METHYLBENZENESULFONAMIDE synthesis
- Product Name:N-(4-METHOXYBENZYL)-N-METHYLBENZENESULFONAMIDE
- CAS Number:915916-89-1
- Molecular formula:C15H17NO3S
- Molecular Weight:291.37
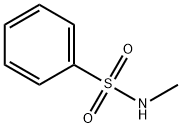
5183-78-8
94 suppliers
$21.00/1g
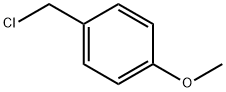
824-94-2
450 suppliers
$24.19/5G

915916-89-1
17 suppliers
$80.00/1g
Yield:915916-89-1 84%
Reaction Conditions:
with sodium t-butanolate in dimethyl sulfoxide at 20; for 3 h;
Steps:
Procedure for N-p-methoxybenzylation using NaOt-Bu/DMSO system (Table 3)
General procedure: To a mixture of amide, imide, sulfonamide, or imidazole (1.0 mmol, 1.0 equiv.) and sodium tert-butoxide (115 mg, 1.2 mmol, 1.2 equiv.) in DMSO (2.0 mL) was added PMBCl (149 mL, 1.1 mmol, 1.1 equiv.). The resulting mixture was stirred at room temperature for 3 h. Upon complete consumption of the starting material, as judged by TLC, the reaction was quenched by water and extracted with AcOEt. The organic layer was dried over Na2SO4, filtered and concentrated in vacuo to give a residue. The residue was purified by silica gel flash column chromatography or MPLC using n-hexane/AcOEt as eluent.
References:
Hamada, Shohei;Sugimoto, Koichi;Iida, Masashi;Furuta, Takumi [Tetrahedron Letters,2019,vol. 60,# 48,art. no. 151277] Location in patent:supporting information
35144-39-9
42 suppliers
$497.00/1g

915916-89-1
17 suppliers
$80.00/1g