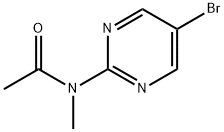
N-(5-BroMopyriMidin-2-yl)-N-MethylacetaMide synthesis
- Product Name:N-(5-BroMopyriMidin-2-yl)-N-MethylacetaMide
- CAS Number:180530-01-2
- Molecular formula:C7H8BrN3O
- Molecular Weight:230.06
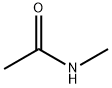
79-16-3
323 suppliers
$8.00/25g
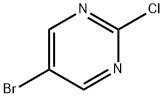
32779-36-5
636 suppliers
$5.00/5g

180530-01-2
9 suppliers
$65.00/100mg
Yield:-
Reaction Conditions:
Stage #1: 5-Bromo-2-chloropyrimidinewith sodium hydride in tetrahydrofuran;mineral oil; for 0.333333 h;
Stage #2: N-methyl-acetamide in tetrahydrofuran;mineral oil;
Steps:
76
To a solution of 5-bromo-2-chloroprimidine (2.00 g, 10.34 mmol) in THF was added 60% NaH (0.34 g, 14.00 mmol) in mineral oil. After 20 minutes, iV-methylacetamide (0.80 g, 11.00 mmol) was added dropwise. After 2 hours, the reaction was diluted with water and extracted with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate and concentrated in vacuo. The residue was purified by silica gel chromatography eluting with 30% ethyl acetate in heptane. The major fractions were combined and the solvent was concentrated in vacuo to afford iV-(5-bromo- pyrimidin-2-yl)-iV-methyl-acetamide.
References:
WO2010/36632,2010,A1 Location in patent:Page/Page column 219