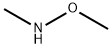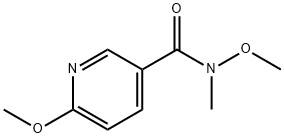
N,6-Dimethoxy-N-Methylnicotinamide synthesis
- Product Name:N,6-Dimethoxy-N-Methylnicotinamide
- CAS Number:858600-08-5
- Molecular formula:C9H12N2O3
- Molecular Weight:196.2
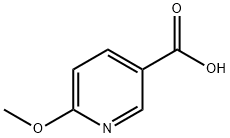
66572-55-2
175 suppliers
$15.00/5g

6638-79-5
594 suppliers
$6.00/25g

858600-08-5
19 suppliers
inquiry
Yield:858600-08-5 100%
Reaction Conditions:
with benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;triethylamine in dichloromethane at 20; for 16 h;
Steps:
22.1 1)
N,O-Dimethylhydroxylamine hydrochloride (11.5 g), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (41.0 g), 1-hydroxybenzotriazole (14.5 g), and triethylamine (54 mL) were added at room temperature to the obtained 6-methoxynicotinic acid (15.0 g) in dichloromethane (600 mL), followed by stirring for 16 hours. The reaction mixture was partitioned between water and dichloromethane. The aqueous layer was extracted with dichloromethane, and then the organic layers were combined. The combined organic layer was washed with saturated brine, followed by drying over sodium sulfate anhydrate. After a filtration step, the solvent was evaporated under reduced pressure, and the residue was purified by silica gel column chromatography (acetone - chloroform), to thereby give 6-methoxynicotinic acid N-methoxy-N-methylamide as an oily product (19.2 g, quantitative amount). (ESI-MS m/z:197(M+H)+).
References:
EP1762568,2007,A1 Location in patent:Page/Page column 41
26218-80-4
164 suppliers
$7.00/1g

6638-79-5
594 suppliers
$6.00/25g

858600-08-5
19 suppliers
inquiry
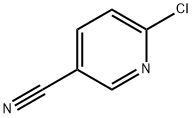
33252-28-7
441 suppliers
$6.00/1g

858600-08-5
19 suppliers
inquiry
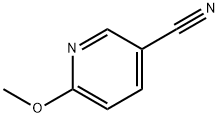
15871-85-9
137 suppliers
$6.00/250mg

858600-08-5
19 suppliers
inquiry