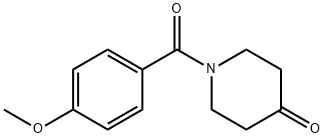
N-Anisoyl-4-piperidone synthesis
- Product Name:N-Anisoyl-4-piperidone
- CAS Number:91586-26-4
- Molecular formula:C13H15NO3
- Molecular Weight:233.26
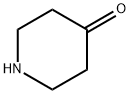
41661-47-6
0 suppliers
$45.00/250mg
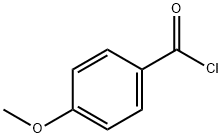
100-07-2
384 suppliers
$35.00/25g

91586-26-4
18 suppliers
inquiry
Yield:91586-26-4 82.2%
Reaction Conditions:
with triethylamine in dichloromethane at 25; for 1 h;
Steps:
6.2 Preparation of 1-(4-methoxybenzoyl)piperidin-4-one
4-Methoxybenzoyl chloride (1.6 g, 9.4 moles)Dissolved in dichloromethane (15 mL),Ice bath, add triethylamine (1.018,1011111101)And 4-4-piperidone(0.998,1011111101),Rise to room temperature (25 ° (:) reaction for one hourAfter completion of the reaction, 100 mL of dichloromethane was added and the mixture was washed with 100 mL of water. The organic phase was dried over anhydrous sodium sulfate and concentrated. The product 1.818 was isolated by column chromatography (PE: ETA = 5: 1) in 82.2% yield.
References:
CN107226808,2017,A Location in patent:Paragraph 0238; 0242-0244
41979-39-9
0 suppliers
$15.00/10mg

100-07-2
384 suppliers
$35.00/25g

91586-26-4
18 suppliers
inquiry
![1,4-dioxa-8-azaspiro[4.5]decan-8-yl-(4-methoxyphenyl)methanone](/CAS/20180723/GIF/190012-08-9.gif)
190012-08-9
1 suppliers
inquiry

91586-26-4
18 suppliers
inquiry

100-07-2
384 suppliers
$35.00/25g

91586-26-4
18 suppliers
inquiry
![1,4-Dioxa-8-azaspiro[4.5]decane](/CAS/GIF/177-11-7.gif)
177-11-7
265 suppliers
$6.00/1g

91586-26-4
18 suppliers
inquiry