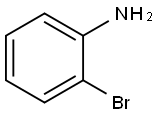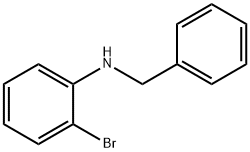
N-benzyl-2-bromoaniline synthesis
- Product Name:N-benzyl-2-bromoaniline
- CAS Number:71687-81-5
- Molecular formula:C13H12BrN
- Molecular Weight:262.15
70787-27-8
6 suppliers
inquiry

71687-81-5
16 suppliers
inquiry
Yield: 48%
Reaction Conditions:
with borane-THF in tetrahydrofuran at 23; for 12 h;Inert atmosphere;
Steps:
General Procedure IV: Reduction of the Anilide
General procedure: The appropriate anilide S1-6 or benzylanilide S7-11 was weighed into an oven-dried, 25-mL, round-bottomed flask. The headspace was purged with argon or N2 and the flask was cappedwith a septum. The anilide was dissolved in a small volume of anhydrous THF (2 - 4 mL), and a1.0M BH3·THF solution (2.0 equiv) was added, resulting in modest warming. The reaction mixturewas stirred at a given temperature for a specified time, after which it was diluted with Et2O (10mL) and quenched by the careful addition of sat. aq. NaHCO3 solution (2 mL). This mixture wasstirred for 1 h, after which it was further diluted with Et2O (30 mL). The mixture was transferredto a 60-mL separatory funnel and washed with sat. aq. sodium potassium tartrate solution (30 mL),10% aq. Na2CO3 solution (30 mL), and brine (30 mL). The organic phase was dried over anhydrous Na2SO4 (~2 g), filtered, and concentrated on a rotavap (30 °C, 15 mm Hg). The resulting crudeproduct was purified by recrystallization, silica gel chromatography, or both, as reported.
References:
Barraza, Scott J.;Denmark, Scott E. [Synlett,2017,vol. 28,# 20,p. 2891 - 2895] Location in patent:supporting information
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

71687-81-5
16 suppliers
inquiry