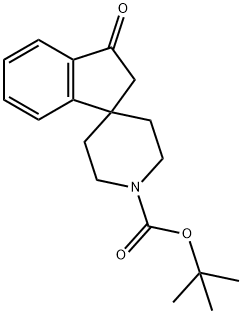
N-BOC-1-[4-SPIRO-PIPERIDINE]-3-INDANONE synthesis
- Product Name:N-BOC-1-[4-SPIRO-PIPERIDINE]-3-INDANONE
- CAS Number:159634-59-0
- Molecular formula:C18H23NO3
- Molecular Weight:301.38
![TERT-BUTYL 3-OXOSPIRO[INDAN-1,4'-PIPERIDINE]-1'-CARBOXYLATE](/CAS/GIF/185525-42-2.gif)
185525-42-2
![N-BOC-1-[4-SPIRO-PIPERIDINE]-3-INDANONE](/CAS/GIF/159634-59-0.gif)
159634-59-0
Tert-butyl 3-hydroxyspiro[indene-1,4'-piperidine]-1'-carboxylate (20 g, 66 mmol) was used as a raw material, which was dissolved in ethyl acetate (300 mL) and o-iodobenzoic acid (IBX) (37 g, 132 mmol) was added with stirring. The reaction mixture was heated to 80 °C for 12 hours. After completion of the reaction, the mixture was cooled to room temperature and the solid was separated by filtration. The filter cake was washed well with ethyl acetate (3 x 100 mL). The filtrate and washings were combined and concentrated under reduced pressure to give a solid residue. The residue was purified by silica gel column chromatography, first eluting with a 1:10 solvent mixture of ethyl acetate-hexane, and then switching to a 1:3 solvent mixture of ethyl acetate-hexane, to give 1'-(tert-butoxycarbonyl)-spiro-(indene-1,4'-piperidinium) (19.5 g, 98% yield) as a white solid with a melting point of 121 °C. The residue was purified by IR (KBr). The product was characterized by IR (KBr), 1H NMR (CDCl3,400 MHz), 13C NMR (CDCl3,100 MHz) and GC-MS (EI) to confirm its structure.
137419-24-0
76 suppliers
$45.00/50mg
![N-BOC-1-[4-SPIRO-PIPERIDINE]-3-INDANONE](/CAS/GIF/159634-59-0.gif)
159634-59-0
87 suppliers
$60.00/25 mg
Yield: 78.7%
Reaction Conditions:
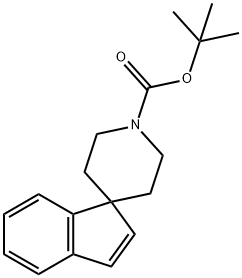
Stage #1:1'-(tert-butyloxycarbonyl)spiro<1H-indene-1,4'-piperidine> with 9-bora-bicyclo[3.3.1]nonane in tetrahydrofuran at 20; for 24 h;Inert atmosphere;
Stage #2: with pyridinium chlorochromate in dichloromethane at 0;Reflux;
Steps:
13.1
Under an argon atmosphere, a solution of t-butyl spiro(indene-1,4'-piperidine)-1'-carboxylate (514 mg, 1.80 mmol) in tetrahydrofuran (5 mL) was added with a solution of 9-borabicyclo[3.3.1]nonane in tetrahydrofuran (0.5 M, 10.5 mL) at room temperature, and the resultant was stirred at the same temperature for 24 hours. The reaction solution was concentrated in vacuo, dissolved into dichloromethane (8 mL), and added with pyridinium chlorochromate (2.30 g, 10.7 mmol) at 0° C. The resultant was stirred for 2 hours by heating under reflux. The reaction solution was filtered using celite, and concentrated in vacuo. The resultant residue was purified using silica-gel chromatography (hexane:ethylacetate=4:1), and t-butyl 3-oxo-2,3-dihydrospriro(indene-1,4'-piperidine-1'-carboxylate (23.5 mg, 78.7%) was obtained as a colorless oil.1H-NMR (400 MHz, CDCl3) δ; 1.46-1.57 (m, 2H), 1.50 (s, 9H), 1.96-2.01 (m, 2H), 2.64 (s, 2H), 2.80-2.89 (m, 2H), 4.20-4.26 (br, 2H), 7.42 (t, J=7.3 Hz, 1H), 7.49 (d, J=7.3 Hz, 1H), 7.65 (t, J=7.3 Hz, 1H), 7.74 (d, J=7.3 Hz, 1H).
References:
KOWA COMPANY, LTD. US2010/22572, 2010, A1 Location in patent:Page/Page column 17-18
![TERT-BUTYL 3-OXOSPIRO[INDAN-1,4'-PIPERIDINE]-1'-CARBOXYLATE](/CAS/GIF/185525-42-2.gif)
185525-42-2
22 suppliers
inquiry
![N-BOC-1-[4-SPIRO-PIPERIDINE]-3-INDANONE](/CAS/GIF/159634-59-0.gif)
159634-59-0
87 suppliers
$60.00/25 mg

137419-24-0
76 suppliers
$45.00/50mg
![N-BOC-1-[4-SPIRO-PIPERIDINE]-3-INDANONE](/CAS/GIF/159634-59-0.gif)
159634-59-0
87 suppliers
$60.00/25 mg
![tert-Butyl 2-oxo-2,3-dihydrospiro[indene-1,4'-piperidine]-1'-carboxylate](/CAS/20180629/GIF/241819-85-2.gif)
241819-85-2
31 suppliers
$36.00/100mg
13292-87-0
245 suppliers
$18.19/5ml

137419-24-0
76 suppliers
$45.00/50mg
![N-BOC-1-[4-SPIRO-PIPERIDINE]-3-INDANONE](/CAS/GIF/159634-59-0.gif)
159634-59-0
87 suppliers
$60.00/25 mg
![tert-Butyl 2-oxo-2,3-dihydrospiro[indene-1,4'-piperidine]-1'-carboxylate](/CAS/20180629/GIF/241819-85-2.gif)
241819-85-2
31 suppliers
$36.00/100mg
118753-70-1
178 suppliers
$6.00/1g
![N-BOC-1-[4-SPIRO-PIPERIDINE]-3-INDANONE](/CAS/GIF/159634-59-0.gif)
159634-59-0
87 suppliers
$60.00/25 mg
![tert-Butyl 2-oxo-2,3-dihydrospiro[indene-1,4'-piperidine]-1'-carboxylate](/CAS/20180629/GIF/241819-85-2.gif)
241819-85-2
31 suppliers
$36.00/100mg