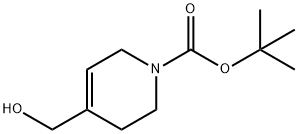
N-Boc-4-(hydroxyMethyl)-1,2,3,6-tetrahydropyridine synthesis
- Product Name:N-Boc-4-(hydroxyMethyl)-1,2,3,6-tetrahydropyridine
- CAS Number:203663-26-7
- Molecular formula:C11H19NO3
- Molecular Weight:213.27
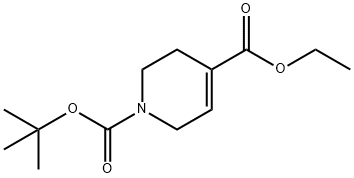
906663-30-7
3 suppliers
inquiry

203663-26-7
34 suppliers
$240.00/25mg
Yield:203663-26-7 87 %
Reaction Conditions:
with diisobutylaluminium hydride in tetrahydrofuran at -78 - 20;Inert atmosphere;
Steps:
Diisobutylaluminum hydride (47.6 mL, 47.6 mmol, 3.2 eq.) was added dropwise to a stirring solution of 1 -(tert-butyl) 4-ethyl 3,6-dihydropyridine- l,4(2H)-dicarboxylate (3.8 g, 14.88 mmol, 1.0 eq.) in THF ( 150 mL) at -78°C under argon atmosphere. The reaction mixture was allowed to stirr at -78°C for 30 min then was allowed to warm up to rt and was kept under stirring for 20 additional minutes. At this point TLC analysis showed full reduction of the ester into the corresponding alcohol (more polar spot, Rf=0.22 in hexane/AcOEt 7:3). 50 mL of NH4CI were slowly added to the reaction mixture at 0 °C and stirring was kept for 3h. The reaction mixture was then filtrated through a pad of celite. The celite cake was washed several times with EtOAc. The aqueous layer was extracted by AcOEt (3x30 mL). Organic layers were combined, washed once with brine (50 mL) and dried over Na2S04. Removal of the solvent under reduced pressure afforded tert-butyl 4- (hydroxymethyl)-3,6-dihydropyridine-l (2H)-carboxylate as a yellow oil (2.77 g, 87%).
References:
WO2020/106627,2020,A1 Location in patent:Paragraph 00324
![1-OXA-6-AZASPIRO[2.5]OCTANE-6-CARBOXYLIC ACID, 1,1-DIMETHYLETHYL ESTER](/CAS/GIF/147804-30-6.gif)
147804-30-6
207 suppliers
$6.00/250mg

203663-26-7
34 suppliers
$240.00/25mg
184368-74-9
50 suppliers
$82.00/100mg

203663-26-7
34 suppliers
$240.00/25mg

79099-07-3
0 suppliers
$5.00/5g

203663-26-7
34 suppliers
$240.00/25mg
159635-49-1
17 suppliers
$5.00/250mg

203663-26-7
34 suppliers
$240.00/25mg