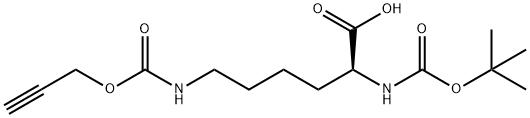
N-α-Boc-propargyl-lysine-OH synthesis
- Product Name:N-α-Boc-propargyl-lysine-OH
- CAS Number:1202704-91-3
- Molecular formula:C15H24N2O6
- Molecular Weight:328.36
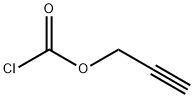
35718-08-2
57 suppliers
$49.29/5ml
13734-28-6
361 suppliers
$8.00/1g

1202704-91-3
9 suppliers
$55.00/10mg
Yield:-
Reaction Conditions:
in tetrahydrofuran;NaOH;ethyl acetate;
Steps:
3 (S)-2-(tert-butoxycarbonylamino)-6-((prop-2-ynyloxy)carbonylamino)hexanoic acid (9)
Boc-Lys-OH (500 mg, 2.03 mmol) was dissolved in 1 M NaOH (5 mL) and THF (5 mL) and cooled to 0° C. Propargyl chloroformate (158.4 mL, 192.5 mg, 1.62 mmol) was added dropwise over 5 minutes and the reaction was allowed to stir for 10 hours at room temperature. The solution was then cooled to 0° C. again, washed with ice-cold Et2O (50 mL), acidified with ice-cold 1 M HCl (50 mL), and was extracted with ice-cold EtOAc (2*30 mL). The combined organic layers were dried over Na2SO4 and the solvents were evaporated to clean give 9 (442 mg, 1.35 mmol) as a white foam in 83% yield. 1H NMR (CDCl3): δ=1.33-1.80 (m, 14H), 2.45 (s, 1H), 3.15 (m, 2H), 4.23 (m, 1H), 4.62-4.68 (m, 2H), 5.25-5.55 (m, 2H), 6.20-6.47 (m, 1H), 11.03 (s, 1H). 13C NMR (CDCl3): δ=22.5, 28.5, 29.3, 32.1, 40.8, 52.6, 53.2, 74.8, 78.5, 80.3, 156.0, 157.3, 176.7. HRMS: m/z calcd for C15H24N2O6 [M+Na]+: 351.15266. found: 351.15245.
References:
US2012/77948,2012,A1