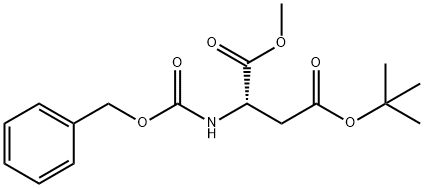
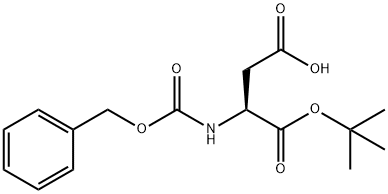
N-Cbz-L-Aspartic acid 4-tert-butyl ester synthesis
- Product Name:N-Cbz-L-Aspartic acid 4-tert-butyl ester
- CAS Number:5545-52-8
- Molecular formula:C16H21NO6
- Molecular Weight:323.34
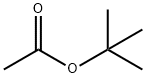
540-88-5
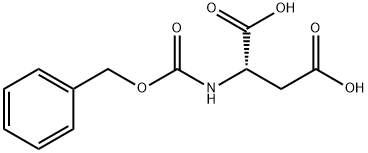
1152-61-0
47307-26-6

5545-52-8
42417-76-5
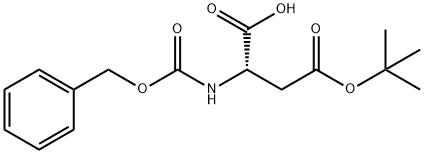
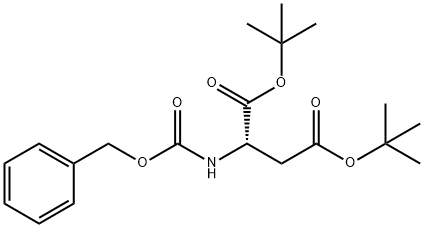
The general procedure for the synthesis of (S)-3-(((benzyloxy)carbonyl)amino)-4-(tert-butoxy)-4-oxobutanoic acid, N-benzyloxycarbonyl-L-aspartic acid 4-tert-butyl ester and Z-L-aspartic acid tert-butyl ester tert-butyl ester, using tert-butyl acetate and N-CBZ-L-aspartic acid, is as follows: an ester-exchange reaction system was set up according to the methodology described in Example 1 , but BF3 -Et2O was replaced with other catalysts. The catalysts tested included acids or salts as listed in Table I. The reaction was carried out at room temperature or at about 50°C for 5.5 to 15 hours. At the end of the reaction, the reaction mixtures were analyzed by HPLC technique as in Example 1. The results of Table I show that the ester exchange reaction in the presence of catalysts such as concentrated sulfuric acid (H2SO4), methanesulfonic acid (MsOH), zinc chloride (ZnCl2), and titanium tetrachloride in dichloromethane (TiCl4 in DCM), significantly produced Z-Asp(OtBu)2. In contrast, the catalytic effect of aluminum chloride (AlCl3), ferric chloride (FeCl3) and titanium tetraisopropoxide (Ti[OiPr]4) was not significant even under the conditions of increasing the reaction temperature and prolonging the reaction time.
![4-Oxazolidineacetic acid, 5-oxo-3-[(phenylmethoxy)carbonyl]-, 1,1-dimethylethyl ester, (4S)-](/CAS/20200119/GIF/23632-69-1.gif)
23632-69-1
0 suppliers
inquiry

5545-52-8
231 suppliers
$11.19/1G