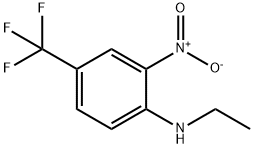
N-ETHYL 2-NITRO-4-(TRIFLUOROMETHYL)ANILINE synthesis
- Product Name:N-ETHYL 2-NITRO-4-(TRIFLUOROMETHYL)ANILINE
- CAS Number:30377-62-9
- Molecular formula:C9H9F3N2O2
- Molecular Weight:234.18
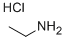
557-66-4
281 suppliers
$26.00/25g
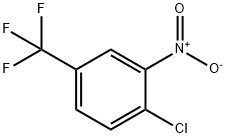
121-17-5
32 suppliers
$19.00/250g

30377-62-9
41 suppliers
$28.60/100mg
Yield:30377-62-9 72%
Reaction Conditions:
with triethylamine in N,N-dimethyl-formamide at 0 - 100; for 2 h;
Steps:
1 Step 1: Synthesis of N-ethyl-2-nitro-4-(trifluoromethyl) aniline
To a stirred solution of l-chloro-2-nitro-4-(trifluoromethyl)benzene (4 g, 17.69, mmol, 1 equiv.) in DMF(28 mL) were added TEA (5.36 mL, 53.07 mmol, 3equiv.) and ethylamine hydrochloride (1.74 g, 21.23 mmol, 1.2 equiv.) portion wise at 0°C. The resulting reaction mixture was stirred at 100°C for 2 h. After completion of reaction (monitored by TLC) was diluted with EtOAc (500 mL), and water (50 mL), The organic layer was separated and washed with brine, dried over anhydrous sodium sulfate and concentrated under vacuum. The crude product was purified by combi-flash eluting with 0-60% EtOAc/hexane to give N-ethyl-2-nitro-4-(trifluoromethyl) aniline (3.0g, 12.81 mmol, 72% yield) as a yellow solid. LC-MS Method A: [M+H]+ = 235.1
References:
WO2022/133098,2022,A2 Location in patent:Page/Page column 95
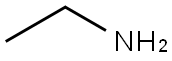
75-04-7
415 suppliers
$10.00/20mg

121-17-5
32 suppliers
$19.00/250g

30377-62-9
41 suppliers
$28.60/100mg