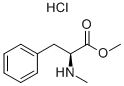
N-ME-PHE-OME HCL synthesis
- Product Name:N-ME-PHE-OME HCL
- CAS Number:19460-86-7
- Molecular formula:C11H16ClNO2
- Molecular Weight:229.7
![L-Phenylalanine, N-[(1,1-dimethylethoxy)carbonyl]-N-methyl-, methyl ester](/CAS/20210305/GIF/37553-64-3.gif)
37553-64-3

19460-86-7
GENERAL PROCEDURE: To a stirring solution of Nα-Boc-protected peptide (1 mmol) in dichloromethane (2 mL) was slowly added a solution of 3.3 M HCl in dioxane (5 mL) at 0 °C. The reaction system was gradually warmed up to 20 °C and maintained at this temperature for 1 hour. Upon completion of the reaction, the solvent was removed by distillation under reduced pressure. The residue was placed in anhydrous ether and stirred to induce precipitation of the crystalline product. For poorly crystalline or strongly hygroscopic compounds, the residue is dissolved in dry chloroform, the solvent is subsequently evaporated, and the product is placed in a vacuum desiccator and dried using P4O10 or KOH as the desiccant. Part of the product needs to be purified by silica gel column chromatography using eluent mixture B or gel filtration through biogel P2 or Fractogel TCK HW 40 followed by lyophilization to obtain the pure product.
![L-Phenylalanine, N-[(1,1-dimethylethoxy)carbonyl]-N-methyl-, methyl ester](/CAS/20210305/GIF/37553-64-3.gif)
37553-64-3
0 suppliers
inquiry

19460-86-7
80 suppliers
$56.00/100MG
Yield:19460-86-7 91.2%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane;dichloromethane at 0 - 20; for 1 h;
Steps:
4.3.1. Cleavage of Nα-Boc group with hydrogen chloride in dioxane
General procedure: A solution of HCl (3.3 M) in dioxane (5 mL) was added to a stirred solution of Nα-Boc-protected peptide (1 mmol) in DCM (2 mL) at 0 °C. The temperature was risen to 20 °C and the solution maintained for 1 h. The solvents were removed under vacuum and the residue stirred in dry ether to produce a crystalline product. In case of the poorly crystallizing or highly hygroscopic compounds the residual material was dissolved in dry chloroform, the solution evaporated and the product dried in a vacuum desiccator over P4O10 or KOH. Some products were purified by chromatography using silica gel and eluent mixture B, or by gel filtration on biogel P2 or fractogel TCK HW 40 with subsequent lyophilization.
References:
Ryakhovsky, Vladimir V.;Ivanov, Andrey S. [Tetrahedron,2012,vol. 68,# 35,p. 7070 - 7076] Location in patent:supporting information; experimental part
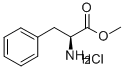
7524-50-7
413 suppliers
$5.00/5g

74-88-4
356 suppliers
$15.00/10g

19460-86-7
80 suppliers
$56.00/100MG

67-56-1
790 suppliers
$9.00/25ml
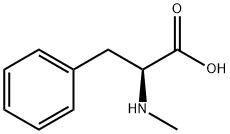
2566-30-5
132 suppliers
$10.00/250mg

19460-86-7
80 suppliers
$56.00/100MG
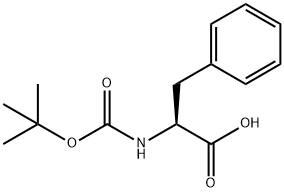
13734-34-4
552 suppliers
$5.50/5g

19460-86-7
80 suppliers
$56.00/100MG