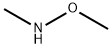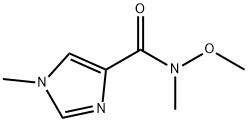
N-methoxy-N,1-dimethyl-1H-imidazole-4-carboxamide synthesis
- Product Name:N-methoxy-N,1-dimethyl-1H-imidazole-4-carboxamide
- CAS Number:873221-68-2
- Molecular formula:C7H11N3O2
- Molecular Weight:169.18

6638-79-5
557 suppliers
$6.00/25g
41716-18-1
221 suppliers
$6.00/250mg

873221-68-2
5 suppliers
inquiry
Yield:873221-68-2 64%
Reaction Conditions:
with benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;triethylamine in N,N-dimethyl-formamide at 20;
Steps:
42.1 1)
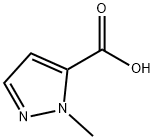
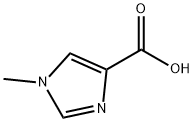
1) N-Methoxy-N-methyl-1-methyl-1H-imidazole-4-carboxamide 1-Methyl-1H-imidazole-4-carboxylic acid (1.26 g), N,O-dimethylhydroxylamine hydrochloride (1.17 g), 1-hydroxybenzotriazole (1.84 g), triethylamine (2.09 mL), and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (2.30 g) were dissolved in N,N-dimethylformamide (50 mL), and the solution was stirred overnight at room temperature. The reaction solvent was evaporated under reduced pressure, and ethanol was added to the residue. Insoluble matter was removed through filtration, and the solvent was evaporated under reduced pressure. The residue was purified through silica gel column chromatography (chloroform - methanol - water, lower phase), to thereby give N-methoxy-N-methyl-1-methyl-1H-imidazole-4-carboxamide as a solid product (1.08 g, 64%). 1H-NMR(400MHz,CDCl3)δ:3.45(3H,s), 3.73(3H,s), 3.78(3H,s), 7.45(1H,s),7.54(1H,s).
References:
EP1762568,2007,A1 Location in patent:Page/Page column 58