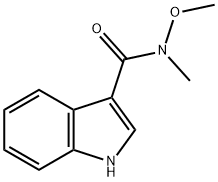
N-METHOXY-N-METHYL-1H-INDOLE-3-CARBOXAMIDE synthesis
- Product Name:N-METHOXY-N-METHYL-1H-INDOLE-3-CARBOXAMIDE
- CAS Number:214759-95-2
- Molecular formula:C11H12N2O2
- Molecular Weight:204.23
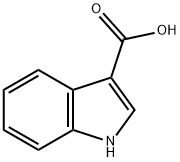
771-50-6
602 suppliers
$5.00/100mg

79-37-8
490 suppliers
$17.67/10gm:

6638-79-5
589 suppliers
$6.00/25g

214759-95-2
11 suppliers
inquiry
Yield:214759-95-2 78%
Reaction Conditions:
with sodium hydrogencarbonate in dichloromethane;triethylamine;
Steps:
F.2.a a
a N-Methoxy-N-methyl-(1H-indol-3-yl)-carboxamide Indole-3-carboxylic acid (2.50 g, 0.0155 mol) was suspended in dichloromethane (60 ml) and treated with oxalyl chloride (1.62 ml, 0.0186 mol), followed by a drop of dry DMF. The reaction mixture was then stirred at room temperature overnight, before being evaporated under reduced pressure and dried in vacuo. The product was then redissolved in dichloromethane (50 ml) and added slowly to a solution of N,O-dimethylhydroxylamine hydrochloride (1.59 g, 0.0163 mol) in dichloromethane (50 ml) containing triethylamine (4.53 ml, 0.0326 mol) under argon. The resultant mixture was then stirred at room temperature for 2 h, before being washed with water, followed by aq. NaHCO3. The organic layer was then dried (Na2 SO4), evaporated under reduced pressure and dried in vacuo to yield the title compound as white solid (2.46 g, 78%). 1 H NMR (200 MHz, CDCl3) δ 9.00 (s, 1H), 8.40 (m, 1H), 7.90 (d, 1H), 7.40 (m, 1H), 7.25 (m, 2H), 3.70 (s, 3H), 3.40 (s, 3H).
References:
US5741801,1998,A
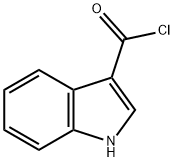
59496-25-2
38 suppliers
$148.00/100mg

6638-79-5
589 suppliers
$6.00/25g

214759-95-2
11 suppliers
inquiry
30030-90-1
36 suppliers
$258.63/1g

214759-95-2
11 suppliers
inquiry
120-72-9
658 suppliers
$6.00/25g

214759-95-2
11 suppliers
inquiry

771-50-6
602 suppliers
$5.00/100mg

214759-95-2
11 suppliers
inquiry