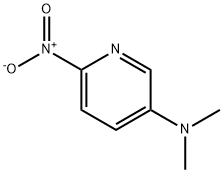
N,N-diMethyl-6-nitropyridin-3-aMine synthesis
- Product Name:N,N-diMethyl-6-nitropyridin-3-aMine
- CAS Number:38772-04-2
- Molecular formula:C7H9N3O2
- Molecular Weight:167.17
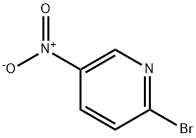
4487-59-6
441 suppliers
$5.00/1g

124-40-3
545 suppliers
$18.00/100ml

38772-04-2
10 suppliers
inquiry
Yield:38772-04-2 76%
Reaction Conditions:
in ethanol at 90; for 72 h;
Steps:
14 Example 14; 2 (R)- (3-Chloro-4-methanesulfonyl-phenyl)-3-cyclopentyl-N- (5-dimethylamino- pyridin-2-yl)-propionamide
Example 14 2 (R)- (3-Chloro-4-methanesulfonyl-phenyl)-3-cyclopentyl-N- (5-dimethylamino- pyridin-2-yl)-propionamide [000167] A sealed tube apparatus was charged with 2-BROMO-5-NITROPYRIDINE (1.02 g, 5.02 mmol) and a 5.6M solution of dimethylamine in ethanol (5.0 mL, 28.0 MMOL). The resulting solution was heated at 90°C for 3 days, allowed to cool to 0°C, and then diluted with acetone and ethyl acetate. A white solid was removed via filtration, and the filtrate was absorbed onto silica gel (Merck Silica gel 60,230-400 mesh). Biotage chromatography (FLASH 40S, Silica, 33-75% ethyl acetate/hexanes) afforded dimethyl- (6-nitro-pyridin-3-yl) -amine (0.64 g, 76%) as an intensely yellow solid: mp 199.8- 200. 5°C ; EI-HRMS m/e calcd for C7H9N302 (M+) 167.0695, found 167.0697.
References:
WO2004/52869,2004,A1 Location in patent:Page 98 - 99
39856-50-3
544 suppliers
$6.00/5g

124-40-3
545 suppliers
$18.00/100ml

38772-04-2
10 suppliers
inquiry