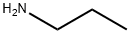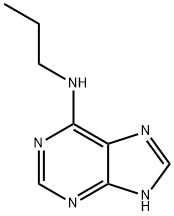
{N}-propyl-9{H}-purin-6-amine synthesis
- Product Name:{N}-propyl-9{H}-purin-6-amine
- CAS Number:16370-58-4
- Molecular formula:C8H11N5
- Molecular Weight:177.21
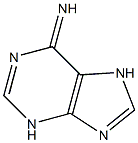
134461-75-9
1 suppliers
inquiry

16370-58-4
2 suppliers
inquiry
Yield:16370-58-4 94.3%
Reaction Conditions:
with hydrogenchloride;propylamine;sodium hydroxide in ethanol;water;
Steps:
16 EXAMPLE 16
EXAMPLE 16 To 350 ml. of ethanol was added 147.8 g. (2.5 moles) of n-propylamine. Into another 350 ml. of ethanol was absorbed 25.5 g. (0.7 mole) of anhydrous hydrogen chloride. The resulting two solutions were put into an autoclave together with 67.5 g. (0.5 mole) of adenine and the reaction was carried out at 160° C. for 24 hours. After completing the reaction, the excess n-propylamine and ethanol were removed by distillation, and 700 ml. of water and then 140 g. (0.7 mole) of a 20% by weight of aqueous solution of sodium hydroxide were added to the residue and the evolved n-propylamine was evaporated under a reduced pressure at 50° C. The residual solution was neutralized with a 49 % by weight sulfuric acid, and the precipitated crystals were filtered and dried at 70° C. for 15 hours to obtain 85.7 g. of a crude N6 -n-propyladenine. The purity was 97.5% by weight and the yield was 94.3%.
References:
US4900826,1990,A
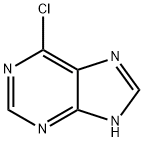
87-42-3
464 suppliers
$14.00/25g

16370-58-4
2 suppliers
inquiry