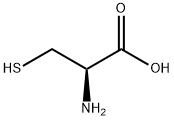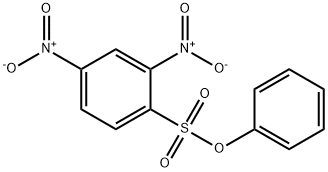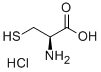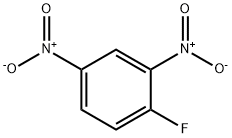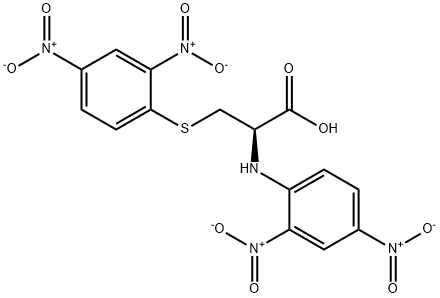
N,S-DI(2,4-DNP)-L-CYSTEINE synthesis
- Product Name:N,S-DI(2,4-DNP)-L-CYSTEINE
- CAS Number:1655-62-5
- Molecular formula:C15H11N5O10S
- Molecular Weight:453.34
Yield:1655-62-5 7%
Reaction Conditions:
with potassium carbonate in water;acetonitrile at 20;
Steps:
2 Synthesis of N,S-bis(2, 4-dinitrophenyl)cysteine (4)
A solution of phenyl 2,4-dinitrobenzenesulfonate (1) (100.0 mg, 0.309 mmol) in 2 mLof CH3CN was added to a mixture of cysteine (18.7 mg, 0.154 mmol) and K2C03 (85.1 mg, 0.616 mmol) in 0.5 mL of deionized water at room temperature. After the reaction was complete, the mixture was concentrated in vacuo. Column purification on silica gel using 100% ethyl acetate followed by 5% formic acid in ethyl acetate as mobile phase afforded 49.9 mg(110 iiMol, 72%) of an amorphous yellow solid. ‘H NIVIR (400 MFIz, (CD3)2C0) = 9.04 (d, J 7.7 Hz, 1H), 8.90 (d, J 2.7 Hz, 1H),8.83 (d, J= 2.5 Hz, 1H), 8.34 (dd, J= 9.0, 2.5 Hz, 1H), 8.29 (dd, J 9.5, 2.7 Hz, 1H),7.38 (d, J 9.5 Hz, 1H), 5.31 (m, 1H), 4.17 (dd, J 14.0, 4.4 Hz, 1H), 4.00 (dd, J=14.0, 6.3 Hz, 1H). ‘3C NIVIR (100 IVIHz, CD3)2C0) = 34.3, 54.5, 115.3, 120.9,123.3, 127.0, 129.2, 130.0, 136.9, 143.3, 144.6, 146.4, 146.8, 146.9, 169.5. SeeFigures 8A-B. (See also Figures 9-13 for COSY, HSQC, HMBC, DEPT, andNOESY spectra, respectively.)
References:
WO2018/200498,2018,A1 Location in patent:Paragraph 0093
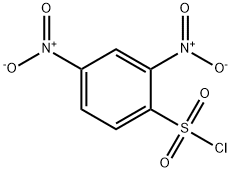
1656-44-6
10 suppliers
$42.60/5g

1655-62-5
9 suppliers
inquiry