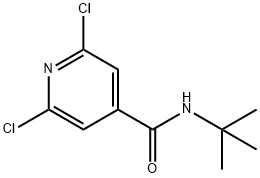
N-tert-butyl-2,6-dichloroisonicotinamide synthesis
- Product Name:N-tert-butyl-2,6-dichloroisonicotinamide
- CAS Number:1152504-12-5
- Molecular formula:C10H12Cl2N2O
- Molecular Weight:247.12
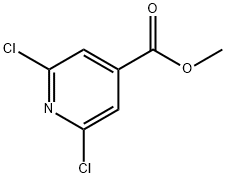
42521-09-5
157 suppliers
$20.00/1g
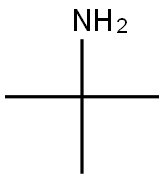
75-64-9
418 suppliers
$10.00/10g

1152504-12-5
9 suppliers
inquiry
Yield:1152504-12-5 93%
Reaction Conditions:
with trimethylaluminum in hexanes;toluene at 0 - 110;
Steps:
7.F
To toluene (48 ml.) at 0 0C is added a solution of Me3AI (19 ml. of 2.0 M in hexanes, 38.8 mmol), followed by dropwise addition of te/f-butylamine (4.1 ml_, 38.8 mmol). The reaction is warmed to rt before 2,6-dichloro-4-methoxycarbonylpyridine (1.0 g, 4.8 mmol) is added. The reaction is heated to 110 0C for 2 h, cooled to 0 0C, and quenched by the slow addition of 1 N HCI. After addition of 30 ml. of 1 N NaOH, the reaction is extracted three times with CH2CI2. The combined organic phases are washed with brine, dried (Na2SO4), and concentrated in vacuo. The residue is purified by flash chromatography (5 to 30% EtOAc/heptanes) to yield 1.1 g (93%) as a white solid: 1H NMR (400 MHz, CDCI3) δ ppm 1.46 (s, 9 H), 5.83 (s, 1 H), 7.51 (s, 2 H).
References:
WO2009/150230,2009,A1 Location in patent:Page/Page column 80-81