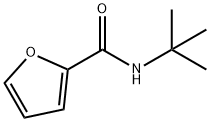
N-tert-butylfuran-2-carboxaMide synthesis
- Product Name:N-tert-butylfuran-2-carboxaMide
- CAS Number:98331-10-3
- Molecular formula:C9H13NO2
- Molecular Weight:167.21
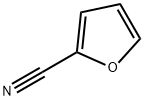
617-90-3
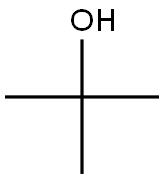
75-65-0

98331-10-3
Under nitrogen atmosphere, 2-cyanofuran (1 mmol) and tert-butanol (2 mmol) were added to a pre oven-dried Schlenk tube. After stirring for 15-20 min at room temperature, a catalytic amount of ionic liquid was added, followed by stirring the reaction mixture at the indicated temperature for a specified time (see Table 1 for specific conditions). The reaction process was monitored by thin layer chromatography (TLC) and gas chromatography-mass spectrometry (GC-MS). Upon completion of the reaction, the reaction mixture was quenched using distilled water followed by neutralization with dilute sodium bicarbonate (NaHCO3) solution. The product was extracted by ether and the organic phase was dried with anhydrous magnesium sulfate (MgSO4) followed by evaporation under vacuum to remove the ether to give the crude product. The crude product was further purified by column chromatography using hexane-ethyl acetate (80:20, v/v) as eluent, resulting in the pure N-(tert-butyl)furan-2-carboxamide as a colorless solid.
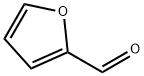
98-01-1
508 suppliers
$24.30/250g
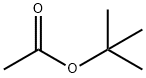
540-88-5
390 suppliers
$24.00/25mL

98331-10-3
20 suppliers
$15.00/5mg
Yield:98331-10-3 94%
Reaction Conditions:
Stage #1: furfuralwith sodium azide;trifluorormethanesulfonic acid;acetic acid at 40; for 2 h;Schmidt Reaction;
Stage #2: acetic acid tert-butyl esterwith trifluorormethanesulfonic acid at 40;Ritter Amidation;
Steps:
General procedure for the syntheses of Substituted N-(tert-butyl)benzamides
General procedure: To a magnetically stirred solution of benzaldehyde (1 mmol) and NaN3 (1.5 mmol) in AcOH (0.2 mL), TfOH (1 mmol) was added and the reaction mixture was heated at 40 °C for 1-2 hrs. On the complete conversion of aldehyde (monitored by TLC) tert-butyl acetate was added alongwith TfOH (1 mmol). The reaction was continued to heating at 40 °C for another 3-5 hrs. After the completion, the reaction was brought to room temperature and ice-cooled aq. sat. NaHCO3 was added to the reaction mixture. The precipitate formed was filtered and washed with plenty of cold water and dried to obtain the desired benzamide as white solid in 96% yield. (Compounds obtained were pure hence, no further purification or recrystallisation was required).
References:
Singh, Garima;Dada, Ravikrishna;Yaragorla, Srinivasarao [Tetrahedron Letters,2016,vol. 57,# 39,p. 4424 - 4427] Location in patent:supporting information
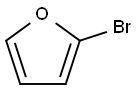
584-12-3
172 suppliers
$20.00/1g
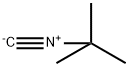
7188-38-7
168 suppliers
$22.39/250MG

98331-10-3
20 suppliers
$15.00/5mg

617-90-3
151 suppliers
$11.00/1g

24424-99-5
863 suppliers
$13.50/25G

98331-10-3
20 suppliers
$15.00/5mg

617-90-3
151 suppliers
$11.00/1g

75-65-0
783 suppliers
$10.00/10ml

98331-10-3
20 suppliers
$15.00/5mg

617-90-3
151 suppliers
$11.00/1g
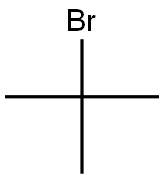
507-19-7
255 suppliers
$10.00/5g

98331-10-3
20 suppliers
$15.00/5mg