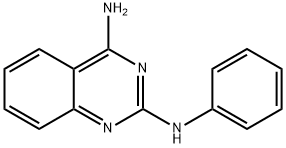
N2-PHENYL-2,4-QUINAZOLINEDIAMINE HYDROCHLORIDE synthesis
- Product Name:N2-PHENYL-2,4-QUINAZOLINEDIAMINE HYDROCHLORIDE
- CAS Number:139308-45-5
- Molecular formula:C14H12N4
- Molecular Weight:236.27
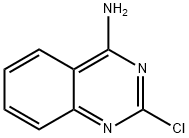
59870-43-8
66 suppliers
$103.00/100mg

62-53-3
689 suppliers
$10.00/1g

139308-45-5
6 suppliers
$60.00/100mg
Yield:139308-45-5 43%
Reaction Conditions:
in ethanol at 80; for 16 h;
Steps:
2 N2-Phenylquinoline-2, 4-diamine (AS224).
A solution of 2-chloroquinazoline-4-amine and aniline was stirred in a pressure tube at 80 °C for 16 h. The solvent was rotary evaporated and the crude material was treated with saturated, aq. NaHCCh, extracted with CH2CI2 (3 x), dried (MgS04) and the solvent was rotary evaporated. The crude was purified by silica gel chromatography (C^Ch/MeOH, 30: 1 v/v) to give AS224 as a colorless solid (8.30 mg, 43%); NMR (600 MHz, DMSO-^e) d 9.96 (br s, 1H), 8.22 (d, J= 8.2 Hz, 1H), 7.94 (br s, 2H), 7.72 - 7.67 (m, 1H), 7.66 - 7.62 (m, 1H), 7.47 - 7.42 (m, 4H), 7.40 - 7.35 (m, 1H), 7.25 - 7.19 (m, 1H), 6.20 (s, 1H); 13C NMR (150 MHz, DMSO-^e) d 156.4, 152.6, 139.4, 138.3, 132.7, 130.0, 125.4, 123.8, 123.7, 123.3, 119.8, 114.9, 86.6; ESI-MS m/z 235.9 [M+H]+; HRMS-ESI (m/z): [M+H]+: calcd. for C15H14N3: 236.1182, found: 236.1180; HPLC: System 1 : tR = 15.5 min, purity 99%, System 2: tR = 14.3 min, purity 99%.
References:
WO2019/204768,2019,A1 Location in patent:Paragraph 0376
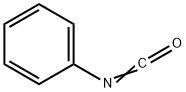
103-71-9
0 suppliers
$20.19/5g

139308-45-5
6 suppliers
$60.00/100mg
![Benzonitrile, 2-[(phenylcarbonimidoyl)amino]-](/CAS/20240320/GIF/99137-18-5.gif)
99137-18-5
0 suppliers
inquiry

139308-45-5
6 suppliers
$60.00/100mg
![Benzonitrile, 2-[(triphenylphosphoranylidene)amino]-](/CAS/20210305/GIF/139308-38-6.gif)
139308-38-6
0 suppliers
inquiry

139308-45-5
6 suppliers
$60.00/100mg
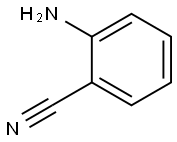
1885-29-6
457 suppliers
$5.00/1g

139308-45-5
6 suppliers
$60.00/100mg