
Naphthalene-1,8-dicarboxaldehyde synthesis
- Product Name:Naphthalene-1,8-dicarboxaldehyde
- CAS Number:17216-14-7
- Molecular formula:C12H8O2
- Molecular Weight:184.19
Yield:-
Reaction Conditions:
with water;ozone in ethyl acetate at 0;Flow reactor;
Steps:
Standard ozonolysis procedure.
General procedure: The syringe pumps and the lm-shear reactorwere primed by running 50 mL EtOAc at 10 mL/min and 50 mL deionized H2O at 10 ml/min with the rotor running at 2000 RPM. This procedure was repeated with pure oxygen also owing at 0.8 L/min The lm-shear reaction chamberwas cooled to 0 C with a recirculator. Both a 0.10 M solution of alkene in EtOAc at 0 °C and deionized water at 0 °C were pumped into a ‘T’ valve (10 mL/min each) generating the plug-ow mixture that entered the film-shear reactor. Ozone was sparged through the reaction chamber at 0.8 L/min. Aproduct ask was primed with 2 mol equiv of a sodium sulte solution toquench any hydrogen peroxide produced. The resulting product mixture was collected in a fume hood (CAUTION residual ozone, formaldehyde, andhydrogen peroxide may be present as an aerosol; perform only in a well ventilated hood). The pH was adjusted to 3 using 3 M HCl (aq) . The two resulting layers were separated. The aqueous layer was washed three times with 15 mLof EtOAc, then the organic layers were combined, dried over Na2SO4, filtered,and the solvent was removed under vacuum. The products were puried asnoted in Supplemental information.
References:
Kendall, Alexander J.;Barry, Justin T.;Seidenkranz, Daniel T.;Ryerson, Ajay;Hiatt, Colin;Salazar, Chase A.;Bryant, Dillon J.;Tyler, David R. [Tetrahedron Letters,2016,vol. 57,# 12,p. 1342 - 1345] Location in patent:supporting information
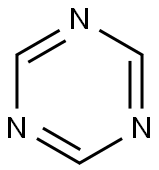
290-87-9
250 suppliers
$9.00/1g
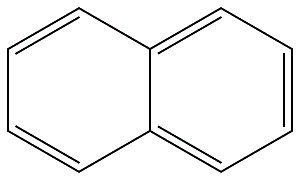
91-20-3
527 suppliers
$14.00/25g

17216-14-7
1 suppliers
inquiry
![Anthra[2,1,9-def:6,5,10-d'e'f']diisoquinoline](/CAS/20200611/GIF/188-97-6.gif)
188-97-6
0 suppliers
inquiry
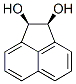
2963-86-2
2 suppliers
inquiry

17216-14-7
1 suppliers
inquiry

91-20-3
527 suppliers
$14.00/25g
201230-82-2
1 suppliers
inquiry

17216-14-7
1 suppliers
inquiry

7141-15-3
3 suppliers
inquiry
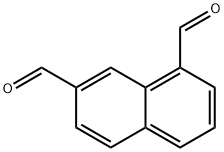
102877-83-8
1 suppliers
inquiry
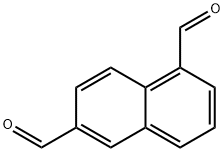
102877-82-7
2 suppliers
inquiry
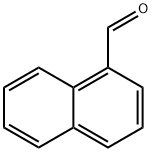
66-77-3
385 suppliers
$7.00/10g
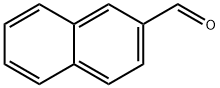
66-99-9
290 suppliers
$5.00/1g
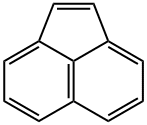
![1,4-Epoxy-1H,4H-naphtho[1,8-de][1,2]dioxepin](/CAS/20200401/GIF/196-15-6.gif)