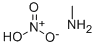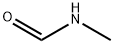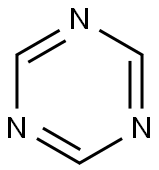
1,3,5-Triazine synthesis
- Product Name:1,3,5-Triazine
- CAS Number:290-87-9
- Molecular formula:C3H3N3
- Molecular Weight:81.08
Amine-substituted triazines called Guanamines are prepared by the condensation of cyanoguanidine with the corresponding nitrile:
(H2N)2C=NCN + RCN → (CNH2)2(CR)N3
Yield:-
Reaction Conditions:
in water for 2 h;
Steps:
N-Methylidenemethylamine was prepared by mixing paraformaldehyde and an aqueous solution of methylamine (40%) with stirring at room temperature for a period of time of 2 hours. The reaction product was extracted with dichloromethane and dried. The reaction product was identified by 1H NMR to be a cyclic trimer.
References:
Loctite (R&D) Limited US7569719, 2009, B1 Location in patent:Page/Page column 10
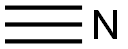
74-90-8
0 suppliers
inquiry

290-87-9
251 suppliers
$9.00/1g
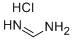
6313-33-3
200 suppliers
$9.00/1g

290-87-9
251 suppliers
$9.00/1g
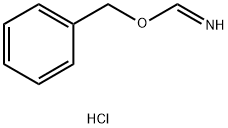
60099-09-4
23 suppliers
inquiry

290-87-9
251 suppliers
$9.00/1g