
NAPHTHO[1,2-B]FURAN-3(2H)-ONE synthesis
- Product Name:NAPHTHO[1,2-B]FURAN-3(2H)-ONE
- CAS Number:58645-78-6
- Molecular formula:C12H8O2
- Molecular Weight:184.19
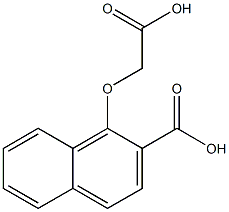
821787-31-9
0 suppliers
inquiry
![NAPHTHO[1,2-B]FURAN-3(2H)-ONE](/StructureFile/ChemBookStructure3/GIF/CB6401803.gif)
58645-78-6
11 suppliers
inquiry
Yield:58645-78-6 48%
Reaction Conditions:
Stage #1: 1-(carboxymethoxy)-2-naphthoic acidwith anhydrous Sodium acetate in acetic anhydride at 150; for 4 h;
Stage #2: with hydrogenchloride in lithium hydroxide monohydrate;
Steps:
34.3
Step 3: The 1-carboxymethoxy-naphthalene-2-carboxylic acid compound obtained from step 2 (12.3 g, 50 mmol) was dissolved in acetic anhydride (100 ml) and anhydrous sodium acetate (10.0 g, excess) was added. The reaction mixture was heated to 150 C. for 4 hrs. During this time the reaction mixture turned dark red. The reaction mixture was cooled to room temperature and quenched carefully with ice cold water. The red solid obtained was filtered and washed well with water. The red solid obtained was suspended in 1 N HCl and refluxed for 2 hrs. A dark red solid, naphtho[1,2-b]furan-3 (2H)-one, precipitated from the reaction mixture. It was filtered and washed well with water. It was dried at 40 C. and used for the next step without further purifications. Yield: 4.5 g (48%); 185 (M+H).
References:
US2005/4162,2005,A1 Location in patent:Page/Page column 19
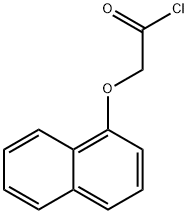
2007-12-7
9 suppliers
$237.00/500mg
![NAPHTHO[1,2-B]FURAN-3(2H)-ONE](/StructureFile/ChemBookStructure3/GIF/CB6401803.gif)
58645-78-6
11 suppliers
inquiry
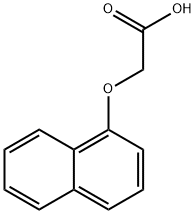
2976-75-2
159 suppliers
$10.00/500mg
![NAPHTHO[1,2-B]FURAN-3(2H)-ONE](/StructureFile/ChemBookStructure3/GIF/CB6401803.gif)
58645-78-6
11 suppliers
inquiry
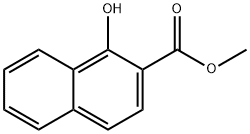
948-03-8
61 suppliers
$168.00/5g
![NAPHTHO[1,2-B]FURAN-3(2H)-ONE](/StructureFile/ChemBookStructure3/GIF/CB6401803.gif)
58645-78-6
11 suppliers
inquiry