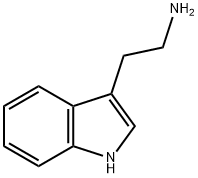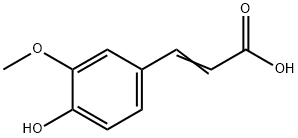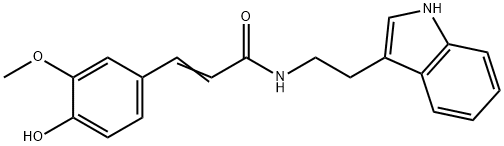
Nb-Feruloyltryptamine synthesis
- Product Name:Nb-Feruloyltryptamine
- CAS Number:53905-13-8
- Molecular formula:C20H20N2O3
- Molecular Weight:336.38
Yield:53905-13-8 20%
Reaction Conditions:
Stage #1: 3-(4-hydroxy-3-methoxyphenyl)acrylic acidwith dicyclohexyl-carbodiimide in acetone at 20; for 1 h;Darkness;
Stage #2: tryptaminewith Sodium hydrogenocarbonate in acetone at 20; for 24 h;Darkness;
Steps:
3.2. General Procedure for the Synthesis of Compounds 1-8
General procedure: All protocols used for optimization of the synthesis are given in the supplementary information (Table S1). This section focusses on the optimized final protocol. To a 0.072 M solution of N,N′-dicyclohexylcarbodiimide (DCC) in acetone, an equimolar amount of hydroxycinnamic acid was added. The solution was stirred for 1 h at room temperature in the dark. Photoisomerization has been reported for several phenol amides,[23,24] therefore exposure to light was limited as much as possible during all syntheses. After 1 h an equimolar amount of the desired amine was added, followed by addition of a 0.072 M sodium bicarbonate solution in a volume equal to the starting volume of acetone. The mixture was stirred for 24 h at room temperature and in the dark. After 24 h the reaction was stopped by adding an equimolar amount of acetic acid. Insoluble N,N′- dicyclohexylurea (DCU) formed was removed by filtration using a cellulose filter. A sample of 200 μL was taken and centrifuged (10,000× g, 5 min) prior to analysis by UHPLCPDA-ESI-IT-MS. For the remaining reaction mixture, acetone was removed under reduced pressure at 40 °C after which the remaining water phase was washed three times with ethyl acetate. For further purification by flash chromatography, the ethyl acetate fraction was used for phenol amides with anthranilate, serotonin, tyramine, and tryptamine subunits. For phenol amides with agmatine or putrescine subunits, the water fraction was used. To the ethyl acetate samples 5 mL water was added prior to concentration. All samples were concentrated under reduced pressure at 60 °C to remove the ethyl acetate before purification by flash chromatography.
References:
Vincken, Jean-Paul;de Bruijn, Wouter J. C.;van Zadelhoff, Annemiek [Molecules,2022,vol. 27,# 7,art. no. 2203]