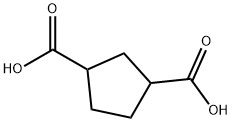
NORCAMPHORICACID synthesis
- Product Name:NORCAMPHORICACID
- CAS Number:4056-78-4
- Molecular formula:C7H10O4
- Molecular Weight:158.15
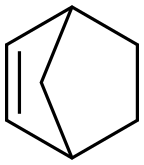
498-66-8

4056-78-4
A mechanical stirrer, J-KEM temperature controller and nitrogen inlet were assembled in a 22 L three-necked round bottom flask. Norbornene (200 g, 2.123 mol), ethyl acetate (1.95 L) and acetonitrile (1.95 L) were added sequentially. The reaction mixture was cooled to 5 °C using an acetone/dry ice bath. Ruthenium trichloride (9.69 g, 46.72 mmol) was added in batches, followed by a slow dropwise addition of an aqueous (2.925 L) suspension of sodium periodate (1.816 kg, 8.707 mol) over 30 min. The reaction became exothermic and was controlled between 10 °C and 15 °C. After 90 min, the reaction mixture thickened significantly, stirring was difficult, and the temperature rose rapidly to 39 °C (exotherm was controlled by replenishing the cooling bath with large amounts of dry ice). The reaction mixture was cooled to 20 °C, the cooling bath was withdrawn and stirring was continued overnight at room temperature. The reaction mixture was filtered through a diatomaceous earth pad to remove solids and the filtrate was concentrated until solids precipitated. The solid was ground with hexane (2 L), filtered and washed with hexane (2 x 500 mL) to give 195 g of cyclopentane-1,3-dicarboxylic acid (58% yield). The product was characterized by 1H NMR (500 MHz, DMSO-d6): δ 12.07 (s, 2H), 2.66-2.73 (m, 2H), 2.06-2.12 (m, 1H), 1.85-1.89 (m, 1H), 1.72-1.85 (m, 4H).
497-38-1
177 suppliers
$13.00/1g

4056-78-4
101 suppliers
$27.00/250mg
Yield:-
Steps:
Multi-step reaction with 3 steps
1: liq. NH3
2: P2O5 / benzene / Heating
3: KMnO4, aq. KOH
References:
Kagawa,M. [Chemical and pharmaceutical bulletin,1961,vol. 9,p. 391 - 403]

498-66-8
235 suppliers
$10.00/5g

4056-78-4
101 suppliers
$27.00/250mg
![2-ethynylbicyclo[2.2.1]heptan-2-ol](/CAS/20200331/GIF/18084-03-2.gif)
18084-03-2
4 suppliers
inquiry

4056-78-4
101 suppliers
$27.00/250mg
![1-bicyclo[2.2.1]hept-2-en-2-ylethan-1-one](/CAS/GIF/71720-43-9.gif)
71720-43-9
3 suppliers
inquiry

4056-78-4
101 suppliers
$27.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

4056-78-4
101 suppliers
$27.00/250mg