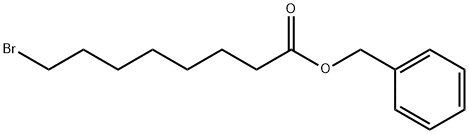
Octanoic acid, 8-bromo-, phenylmethyl ester synthesis
- Product Name:Octanoic acid, 8-bromo-, phenylmethyl ester
- CAS Number:72284-86-7
- Molecular formula:C15H21BrO2
- Molecular Weight:313.23
17696-11-6
320 suppliers
$13.00/1g

100-51-6
1429 suppliers
$5.00/100g

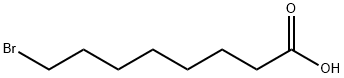
72284-86-7
2 suppliers
inquiry
Yield:72284-86-7 100%
Reaction Conditions:
with 4-pyrrolidin-1-ylpyridine;dicyclohexyl-carbodiimide in dichloromethane at 20;Inert atmosphere;
Steps:
10.10a Preparation of benzyl 8-bromooctanoate
Procedure A Preparation of benzyl esters using carbodiimides; A solution of Ν,Ν'-dicyclohexylcarbodiimide (DCC) or N,N'-diisopropylcarbodiimide in dry dichloromethane ( 0 mL) was added drop-wise via a syringe to a stirred solution of the carboxylic acid, benzyl alcohol or 2,2,2-trichloroethanol, and 4-pyrrolidinylpyridine in dry dichloromethane (40 mL) under argon. After stirring for one to two days at ambient temperature, the mixture was vacuum filtered and the residue was washed with a little dichloromethane. The solvent was evaporated off and the residue was vacuum dried at 50°C (bath temperature) and 0.014 mm Hg using a Kugelrohr apparatus to remove excess benzyl alcohol. This compound was prepared from 8-bromooctanoic acid (2.23 g; 10.0 mmol), benzyl alcohol (1.19 g; 11.0 mmol), Ν,Ν'-dicyclohexylcarbodiimide (DCC, 2.27 g; 11.0 mmol), and 4-pyrrolidinylpyridine (50 mg) in dry dichloromethane (50 mL) using Procedure A. 3.14 g (100%) product was obtained (clear oil that eventually solidified on standing). H NMR (200 MHz; CDCI3): δ 1.33 (6H, br s), 1.6-1.7 (2H, m), 1.75-1.9 (2H, m), 2.35 (2H, t, J = 7.8 Hz); 3.38 (2H, t, J = 6.8 Hz); 5.11 (s, 2H), 7.35 (s, 5H) ppm. 13C NMR (50 MHz; CDCI3): δ 24.8, 27.9, 28.3, 28.9, 32.6, 33.8, 34.2, 66.0, 128.1 , 128.4, 136.0, 173.3 ppm.
References:
WO2014/20164,2014,A1 Location in patent:Page/Page column 45;
