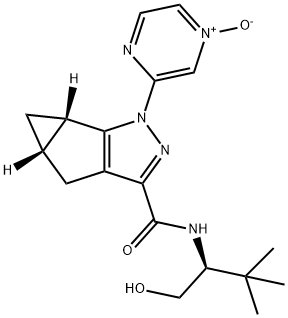
Olorinab synthesis
- Product Name:Olorinab
- CAS Number:1268881-20-4
- Molecular formula:C18H23N5O3
- Molecular Weight:357.41
![(2S,4S)-9-(4-oxidopyrazin-4-ium-2-yl)-8,9-diazatricyclo[4.3.0.02]nona-1(6),7-diene-7-carboxylic acid](/CAS/20210111/GIF/1268883-00-6.gif)
1268883-00-6
10 suppliers
inquiry
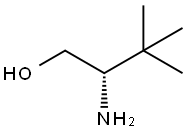
112245-13-3
287 suppliers
$10.00/250mg

1268881-20-4
29 suppliers
inquiry
Yield: 74.9%
Reaction Conditions:
Stage #1:3-((4aS,5aS)-3-carboxy-4,4a,5,5a-tetrahydro-1H-cyclopropa[4,5]cyclopenta[1,2-c]pyrazol-1-yl)pyrazine 1-oxide with triethylamine;N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate in acetonitrile at 0 - 5; for 0.75 h;Large scale;
Stage #2:(S)-tert-leucinol in acetonitrile at 0 - 5;Large scale;
Steps:
6 Example 6: Preparation of (la5,5a5)-2-(4-oxy-pyrazin-2-yl)-la,2,5,5a-tetrahydro-l/ -2,3-diaza- cyclopropa[a]pentalene-4-carboxylic acid ((5)-l-hydroxymethyl-2,2-dimethyl-propyl)-amide (Compound 1, Formula (I)).
Example 6: Preparation of (la5,5a5)-2-(4-oxy-pyrazin-2-yl)-la,2,5,5a-tetrahydro-l/ -2,3-diaza- cyclopropa[a]pentalene-4-carboxylic acid ((5)-l-hydroxymethyl-2,2-dimethyl-propyl)-amide (Compound 1, Formula (I)). To 3-((4alS',5alSr)-3-carboxy-4,4a,5,5a-tetrahydro-l/i-cyclopropa[4,5]cyclopenta[l,2-c]pyrazol- l-yl)pyrazine 1 -oxide (1.62 kg, 6.27 mol) was added acetonitrile (12.75 kg) and the slurry cooled to 0- 5°C. To the mixture was added triethylamine (1.26 kg; 12.5 mol; 2.0 eq.) and the mixture stirred at 0- 5°C for 0.5 hour. To the resulting solution at 0-5°C was added HATU (2.85 kg, 7.50 mol, 1.2 eq.) and the mixture stirred at 0-5°C for 0.25 hour. Thereafter, a solution of (lSr)-2-amino-3,3-dimethylbutan-l-ol (i.e., (S)-ieri-leucinol) (0.87 kg, 7.42 mol, 1.18 eq.) in acetonitrile (7.38 kg) was added all at once. The resulting mixture was stirred at 0-5°C. The progress of the reaction is monitored by HPLC and the reaction was deemed complete when the amount of starting material (i.e., 3-((4alS',5aS')-3-carboxy- 4,4a,5,5a-tetrahydro- l/i-cyclopropa[4,5]cyclopenta[l,2-c]pyrazol-l-yl)pyrazine 1-oxide) was less than 0.5% (area) as determined by HPLC. To the mixture was added concentrated aqueous hydrochloric acid (0.08 kg) in purified water (0.75 kg) and the stirred reactor contents evaporated under reduced pressure at 20-45°C until no appreciable distillate was observed. To the reactor contents was added water (16.23 kg) and the mixture evaporated under reduced pressure at 20-45°C until no appreciable distillate was observed. Thereafter, ethyl acetate (14.61 kg), and potassium carbonate (0.81 kg) dissolved in water (1.62 kg) was added to the reactor contents. The mixture was stirred at room temperature for at least 0.5 hour. The biphasic solution was allowed to settle and the phases separated. The aqueous layer was extracted twice with ethyl acetate (2 x 7.32 kg). The combined ethyl acetate layer was evaporated under reduced pressure at 25-45°C. The mixture was then chased with ethanol (12.9 kg) under reduced pressure at 20-45°C. Thereafter, aqueous potassium carbonate solution (0.32 kg dissolved in 1.62 kg water) was added and the mixture was heated to reflux for 2 hours. The resulting solution was cooled to 35-40°C and water (19.44 kg) was added slowly. The mixture was stirred at 35-40°C for a minimum of 2 hours when solids began to form. Additional water (8.14 kg) was added to the mixture slowly at 35-40°C. Thereafter, the mixture was cooled to 0-5°C and stirred for a minimum of 2 hours and filtered. The filter cake was washed with water (2 x 8.1 kg). The filter cake was transferred to the reactor and dissolved in acetonitrile (3.84 kg)/water (1.62 kg) at 55-65°C. The solution was cooled to 35-40°C and water (8.10 kg) was added slowly. The mixture was stirred at 35-40°C vigorously for a minimum of 2 hours when solids began to form. Additional water (14.58 kg) was slowly added to the mixture at 35-40°C. Thereafter, the mixture was stirred for a minimum of 3 hours at 0-5 °C and filtered. The filter cake was washed with water (2 x 8.1 kg). The resulting filter cake was dried in a vacuum oven at 65°C to a constant weight (i.e., dry time was 40.5 hrs). The yield of (laS,5aS)-2-(4-oxy-pyrazin-2-yl)-la,2,5,5a-tetrahydro-l/i-2,3-diaza- cyclopropa[a]pentalene-4-carboxylic acid ((S)- l-hydroxymethyl-2,2-dimethyl-propyl)-amide (Compound 1) was 1.68 kg (74.9%; two additional batches were prepared in a similar manner as described herein with yields of 72.3% and 70.7% respectively for an average yield for the three batches of 72.6%); HPLC purity 99.8%; enantiomeric purity > 99.9% as determined by the amount of the Compound of Formula (IIIc) present (i.e., < 0.1% (quantitation limit)); water content (Karl Fisher) 0.1 %; for more anal NMR (DMSO- , 400 MHz): δ 0.42 (td, / = 4.4, 7.8 Hz, 1H), 0.93 (s, 9H), 1.22-1.28 (m, 1H), 2.26-2.32 (m, 1H), 2.64-2.67 (m, 1H), 2.77 (d, / = 16.7 Hz, 1H), 2.85 (dd, / = 6.2, 16.6 Hz, 1H), 3.53-3.59 (m, 1H), 3.68-3.73 (m, 1H), 3.81-3.86 (m, 1H), 4.56 (t, / = 5.4 Hz, 1H, OH), 7.69 (d, / = 9.8 Hz, 1H), 8.28 (dd, / = 1.6, 4.2 Hz, 1H), 8.45 (d, / = 4.3 Hz, 1H), 9.08 (d, / = 1.1 Hz, 1H); 13C NMR (DMSO- , 100 MHz) δ 16.88, 16.95, 23.75, 26.01, 26.95, 34.01, 58.63, 60.30, 124.16, 127.84, 132.07, 143.35, 145.78, 151.10, 154.68, 160.89. The PXRD of the isolated product is shown in Figure 7 and conforms to the PXRD for the anhydrous crystalline form of Compound 1 previously reported in International Publication Number WO2012/116276. Representative PXRD peaks for Compound 1 prepared according to Example 6 are shown below in Table 6. Table 6
References:
ARENA PHARMACEUTICALS, INC.;PAL, Biman B.;MONTALBAN, Antonio Garrido WO2016/85941, 2016, A1 Location in patent:Page/Page column 76; 77; 78
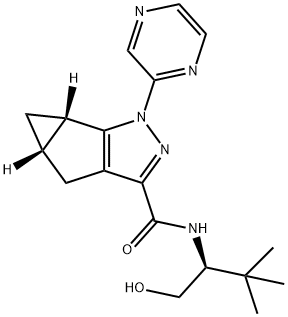
1268881-17-9
0 suppliers
inquiry

1268881-20-4
29 suppliers
inquiry
![Bicyclo[3.1.0]hexan-2-one, (1S,5R)-](/CAS/20180601/GIF/196488-92-3.gif)
196488-92-3
20 suppliers
inquiry

1268881-20-4
29 suppliers
inquiry
![1H-Cyclopropa[4,5]cyclopenta[1,2-c]pyrazole-3-carboxylic acid, 4,4a,5,5a-tetrahydro-1-(2-pyrazinyl)-, ethyl ester, (4aS,5aS)-](/CAS/20210111/GIF/1268883-11-9.gif)
1268883-11-9
0 suppliers
inquiry

1268881-20-4
29 suppliers
inquiry
![1H-Cyclopropa[4,5]cyclopenta[1,2-c]pyrazole-3-carboxylic acid, 4,4a,5,5a-tetrahydro-1-(2-pyrazinyl)-, (4aS,5aS)-](/CAS/20210111/GIF/1268882-99-0.gif)
1268882-99-0
1 suppliers
inquiry

1268881-20-4
29 suppliers
inquiry