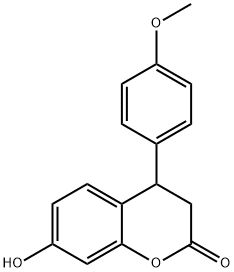
OTAVA-BB BB7020402300 synthesis
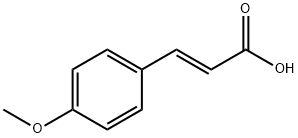
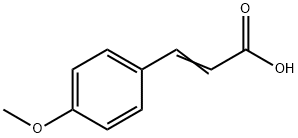
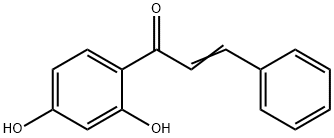
- Product Name:OTAVA-BB BB7020402300
- CAS Number:109386-28-9
- Molecular formula:C16H14O4
- Molecular Weight:270.28
Yield:109386-28-9 79.2%
Reaction Conditions:
with sulfated montmorillonite K-10 in nitrobenzene at 100;Catalytic behavior;Green chemistry;
Steps:
General Procedure for the Synthesis of 4-Aryl-3,4-dihydrocoumarins 9-20
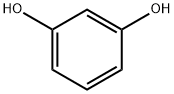
General procedure: A mixture of a substituted cinnamicacid (5 mmol) and a substituted phenol (5 mmol) in nitrobenzene (10 mL) was heated to 100C. Sulfated montmorilloniteK-10 (2 g) was added to the mixture after all reagents were dissolved, and the mixture was stirred at 100C for 3-12 h.The reaction process was monitored by TLC (CH2Cl2-CH3OH, 10:1). The suspension was directly filtered, and a suitableamount of petroleum ether was added to the filtrate. Then the mixture was cooled and stored or stirred under low temperaturefor a certain length of time to promote crystallization of the product. The crude product was recrystallized from EtOAc-PE toafford 9-20.
References:
Li, Na;Wang, Bing;Sun, Jing-yong;Wang, Xiao-jing;Sun, Jie [Chemistry of Natural Compounds,2017,vol. 53,# 5,p. 860 - 865][Khim. Prir. Soedin.,2017,vol. 53,# 5,p. 733 - 736,4]
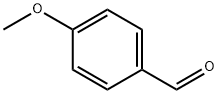
123-11-5
884 suppliers
$10.00/10g

109386-28-9
4 suppliers
inquiry