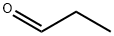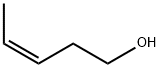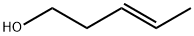
OXY-3-PENTENE synthesis
- Product Name:OXY-3-PENTENE
- CAS Number:764-37-4
- Molecular formula:C5H10O
- Molecular Weight:86.13
Yield:-
Reaction Conditions:
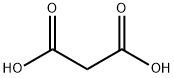
Stage #1:malonic acid;propionaldehyde with piperidine in 5,5-dimethyl-1,3-cyclohexadiene at 40; for 1 h;Dean-Stark;Reflux;Knoevenagel Condensation;
Stage #2: with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 5; for 2 h;Inert atmosphere;Reflux;
Steps:
(E)-3-Pentenoic acid (1): Propanal (18 mL, 0.25 mol) was slowlyadded at 40 °C over 1 h to a mixture of malonic acid (78 g, 0.75 mol),piperidine (0.25 mL, 2.5 mmol) and xylene (100 mL). The reactionapparatus was equipped with a Dean-Stark trap for the continuousremoval of water during the reaction. The reaction temperaturewas raised to reflux after the addition. When no more water wasseparated, the reaction mixture was cooled to room temperature andfiltered to remove the excess malonic acid. Xylene was removed underreduced pressure. The residue was distilled under vacuum (0.4 kPa,60-65 °C) [lit.10 b.p. 66 °C (4 mmHg)] to give (E)-3-pentenoic acid1 as a colourless oil (7.5 g, 30% yield). 1H NMR (CDCl3) δ 1.68 (3H,d, J = 6.0 Hz, Me(5)), 3.05 (2H, d, J = 5.7 Hz, H-C(2)), 5.54 (2H, m,H-C(3) and C(4)), 10-12 (1H, br, -COOH). 13C NMR (CDCl3) δ 17.9(Me), 37.8(C(2)), 121.9 (C(4)), 130.0 (C(3)), 179.0 (C(1)). 1H NMR datawere identical to those reported in the literature (E)-3-Penten-1-ol (2): Lithium aluminium hydride (3.8 g, 0.1 mol)was suspended in dry tetrahydrofuran (50 mL) under nitrogen. (E)-3-Pentenoic acid 1 (10 g, 0.1 mol) dissolved in dry tetrahydrofuran(30 mL) was added dropwise at 0-5 °C. After the addition, thereaction mixture was heated at reflux for 2 h. Then it was cooled to0 °C and quenched by careful addition of distilled water (15 mL) and10% sodium hydroxide solution (10 mL). The mixture was filteredover Na2SO4, and the filtrate was evaporated under reduced pressure(0.4 kPa, 35-40 °C) [lit.11 b.p. 130-138 °C] to give (E)-3-pentenol 2 as ayellow oil (7.6 g, 88% yield). 1H NMR (CDCl3) δ 1.65 (3H, d, J = 6.3 Hz,Me(5)), 2.22 (2H, m, H-C(2)), 3.59 (2H, t, J = 6.3 Hz, H-C(1)), 5.38(1H, m, H-C(4)), 5.57 (1H, m, H-C(3)). 13C NMR (CDCl3) δ18.0 (Me),35.9 (C(2)), 62.0 (C(1)), 127.2 (C(4)), 128.1 (C(3)). The 1H NMR datawere identical with those reported in the literature.
References:
Dai, Yifeng;Sun, Baoguo;Yang, Shaoxiang;Liu, Yongguo;Tian, Hongyu;Shao, Junqiang [Journal of Chemical Research,2014,vol. 38,# 4,p. 236 - 239]
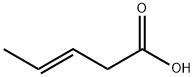
1617-32-9
57 suppliers
$17.67/250mgs:
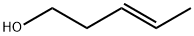
764-37-4
40 suppliers
inquiry
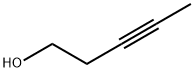
10229-10-4
101 suppliers
$17.00/1g
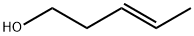
764-37-4
40 suppliers
inquiry
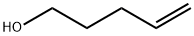
821-09-0
253 suppliers
$21.21/1gm:
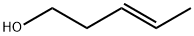
764-37-4
40 suppliers
inquiry