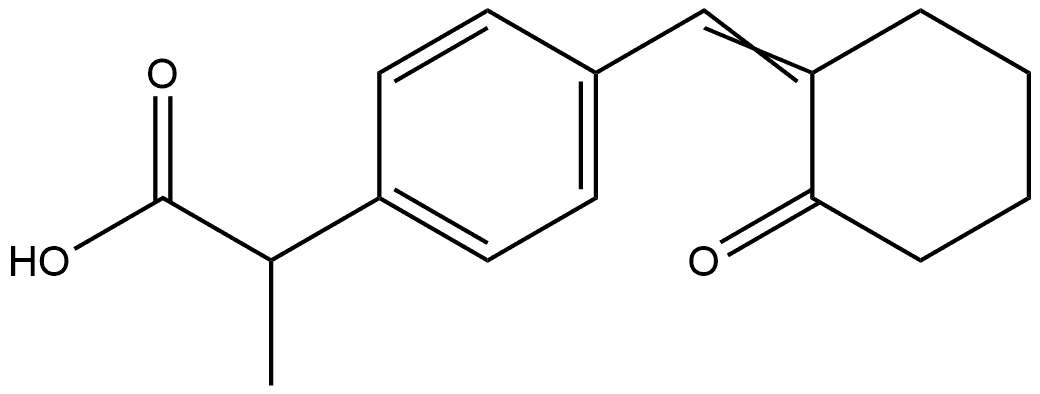
Pelubiprofen Impurity synthesis
- Product Name:Pelubiprofen Impurity
- CAS Number:344338-25-6
- Molecular formula:C16H18O3
- Molecular Weight:258.31
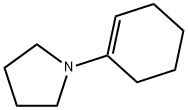
1125-99-1
112 suppliers
$27.93/5g
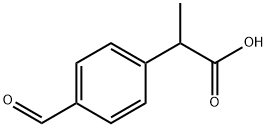
43153-07-7
110 suppliers
$40.00/2mg

344338-25-6
3 suppliers
inquiry
Yield:344338-25-6 97%
Reaction Conditions:
Stage #1: 1-(1-Cyclohexen-1-yl)pyrrolidine;2-(4-formylphenyl)propanoic acid in 1,2-dichloro-ethane at 40; for 6 h;
Stage #2: with hydrogenchloride;water at 20; for 1 h;Solvent;Temperature;
Steps:
1-3 Example 2> Preparation of 2-4-[(2-oxo-cyclohexylidene)methyl]-phenyl-propionic acid (pelubipropene)
Dissolve 5 g (0.028 mole) 2-(4-formylphenyl)propanoic acid and 5.1 g (0.034 mole) 1-pyrrolidinocyclohexene (molar ratio 1:1.2) in a 250 ml reaction vessel in 60 ml dichloroethane and 40 The mixture was stirred for 6 hours while heating. After the reaction was completed, the reaction mixture was cooled to room temperature, and then 30 ml 6N hydrochloric acid solution was slowly added, followed by stirring at room temperature for 1 hour. 30 ml of water was added to the mixture, 50 ml of dichloroethane was further added to separate the layers, and the organic layer was recovered. The aqueous layer was re-extracted with 30 ml of dichloromethane, and the organic layer was collected and washed with 50 ml of water. After drying the organic layer with sodium sulfate (Na2SO4) 10 g, the organic layer was concentrated under reduced pressure at a bath temperature of 30°C. The concentrated residue was recrystallized with 20 ml of a 1:1 mixed solvent of ethyl acetate and hexane to obtain 7.0 g (0.027 mole; yield 97%) of a pure felubiprofen product.
References:
KR2020/69617,2020,A Location in patent:Paragraph 0072-00880

43153-07-7
110 suppliers
$40.00/2mg
108-94-1
523 suppliers
$12.00/50g

344338-25-6
3 suppliers
inquiry
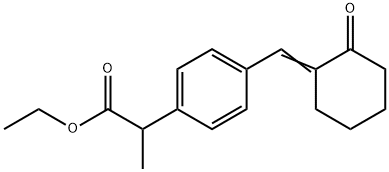
69956-74-7
1 suppliers
inquiry

344338-25-6
3 suppliers
inquiry
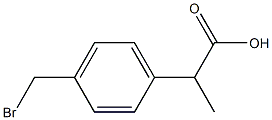
111128-12-2
459 suppliers
$5.00/5g

344338-25-6
3 suppliers
inquiry
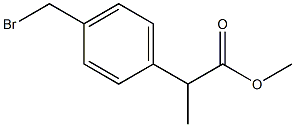
99807-54-2
35 suppliers
inquiry

344338-25-6
3 suppliers
inquiry